| |
Adjuvant Hormonal Therapy |
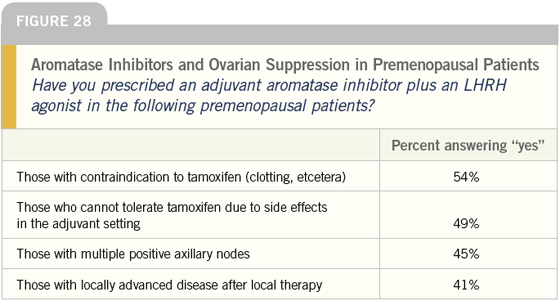
DR LOVE: Joyce, what are your thoughts about using LHRH agonists plus aromatase inhibitors as adjuvant therapy off protocol in premenopausal women (Figure 28)?
DR O’SHAUGHNESSY: I have combined an LHRH agonist with an aromatase inhibitor but it’s rare because for women that I consider high enough risk for that therapy — multiple positive nodes or even node-positive, HER2-positive breast cancer — I generally recommend oophorectomy and then I’m comfortable with an aromatase inhibitor.
DR LOVE: Do you think the responses would have been different if we replaced LHRH agonists with oophorectomy in this question?
DR O’SHAUGHNESSY: Yes. We have little data on LHRH agonists and aromatase inhibitors. Robertson and his colleagues reported on a small, 16-patient study in metastatic breast cancer. The Austrian Breast Cancer Study Group has some data regarding estrogen levels in the adjuvant setting, but we have no efficacy data. The problem with the LHRH agonists is that they’re a little “squirrelly” in their pharmacodynamics and how long they last.
I find that when I use the every threemonth depo-goserelin, it doesn’t always last a full three months, so I give it routinely every 10 or 11 weeks. At that point patients begin noticing premenstrual symptoms, but after they receive the injection they experience their menopausal symptoms all over again.
I don’t find that to be the case on the every four-week goserelin, which was approved by the FDA based on good pharmacodynamic data of estrogen suppression over a four-week period. I don’t have much experience with leuprolide acetate because it’s not approved for breast cancer and reimbursement can be difficult, so I use goserelin.
Occasionally I see a young patient who has not yet had children, so I use an LHRH agonist plus an aromatase inhibitor for four or five years. Then I stop treatment and allow her ovaries to recover to give her a chance at having children, but that’s a rare scenario.
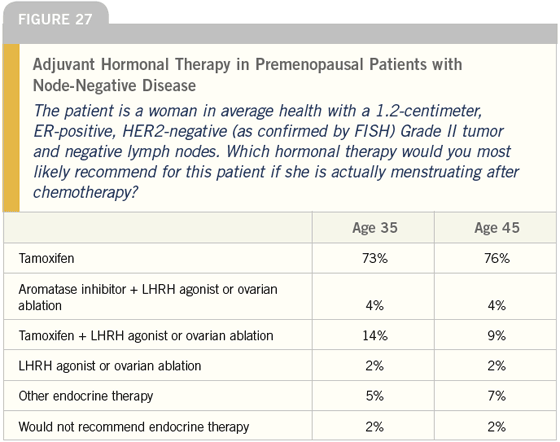
DR LOVE: Which hormonal therapy would you most likely use in this situation (Figure 27)?
DR O’SHAUGHNESSY: In addition to the information provided, to decide between an aromatase inhibitor and tamoxifen, I would consider the percent of cells staining positive for ER and/or PR, the intensity of the staining and the Ki-67.
Patients with a Ki-67 of less than five percent may not relapse for 12 years; however, this tumor is Grade II, so the Ki-67 is probably midrange and if the patient is premenopausal, I would use tamoxifen.
DR LOVE: What about the patient with a HER2-positive tumor?
DR O’SHAUGHNESSY: In this premenopausal patient, I would use tamoxifen. In these patients, the estrogen levels are high and the estrogen is acting as a cofactor with the HER2. I imagine the ER in the plasma membrane or the cell membrane cross-talking with the HER2 and when that ligand is occupied by either tamoxifen or estrogen, that can cross-talk with that growth factor signaling pathway.
I believe tamoxifen is beneficial in premenopausal women with HER2- positive disease; however, I worry about tamoxifen in postmenopausal women with HER2-positive breast cancer. I believe tamoxifen has a greater likelihood of acting as an agonist in that scenario.
DR LOVE: And you, Bob?
DR CARLSON: My practice patterns are consistent with the majority of respondents and I tend to use tamoxifen as a single agent in premenopausal women with estrogen receptor-positive disease.
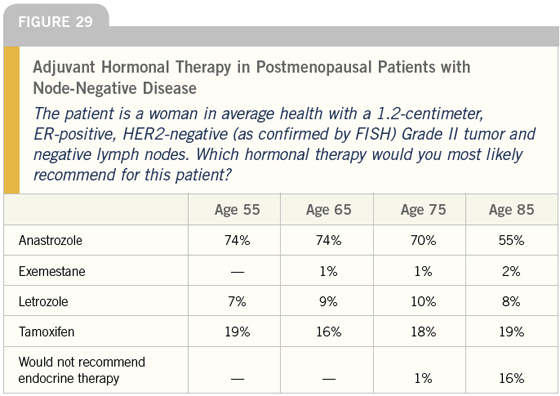
DR LOVE: What about the postmenopausal patient, Joyce (Figure 29)?
DR O’SHAUGHNESSY: In that scenario, I use aromatase inhibitors — mostly anastrozole. However, I find that patients who were peri- or premenopausal before chemotherapy and have recently become postmenopausal, or who have recently stopped hormonereplacement therapy after a long period of use, are the patients who suffer from arthralgias while on aromatase inhibitors. I have no problem giving them two years of tamoxifen and then switching to an aromatase inhibitor.
DR LOVE: How long do you continue the aromatase inhibitors?
DR O’SHAUGHNESSY: Five years. We participate in the ATAC trial and that is an ongoing trial. Some of my patients are just completing their five years of therapy and, if they are at very high risk, I’m advising them to continue the anastrozole until we know more.
DR LOVE: Bob?
DR CARLSON: With the majority of postmenopausal patients, I too tend to use an aromatase inhibitor, generally anastrozole, in the adjuvant setting. If a contraindication or resistance to using an aromatase inhibitor exists, my second option is tamoxifen. I guess I’m surprised that today so many postmenopausal women are receiving an aromatase inhibitor as first-line adjuvant hormonal therapy. That is a huge shift from what we saw just a couple of years ago.
I’m also somewhat surprised that aromatase inhibitors seem to be used less as patients reach the age of 85. I would have expected the opposite.
DR LOVE: It looks like that shift is from anastrozole to no treatment. Only 55 percent of oncologists said they would use anastrozole to treat an 85-year-old woman?
DR CARLSON: That surprises me because I generally recommend hormonal therapy and use anastrozole unless the patient has substantial comorbidities. The patient’s expected survival would have to be quite short to make me think the mild toxicity of the hormonal therapy wasn’t warranted.
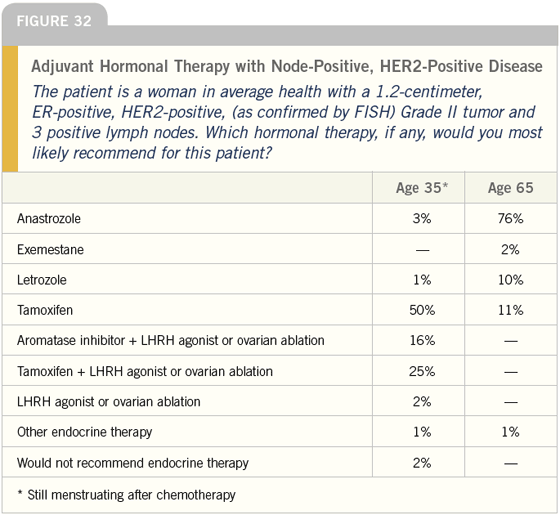
DR LOVE: Joyce, what do you tend to do for women with ER-positive, HER2- positive disease? Do you take into account HER2 status when you make a decision about adjuvant hormonal therapy (Figures 30-32)?
DR O’SHAUGHNESSY: Yes, I do. I use an aromatase inhibitor for postmenopausal patients with ER-positive, HER2-positive disease. I feel strongly about that mainly based on the small amount of preoperative data we have from IMPACT and Matt Ellis’ work.
In premenopausal women, this is much more complicated. I saw an interesting poster at ASCO from Kent Osborne’s group. They burdened animals with human breast cancer cell lines that were ER-positive and HER2-positive.
They then treated the animals with tamoxifen, which had a stimulatory effect in the absence of exogenous estradiol pellets. What I thought I saw in that preclinical work was, when they made these animals premenopausal by giving them estrogen pellets, the tamoxifen was inhibitory. This suggests that it depends on the estrogen milieu of the patient as to whether tamoxifen will be an agonist or an antagonist.
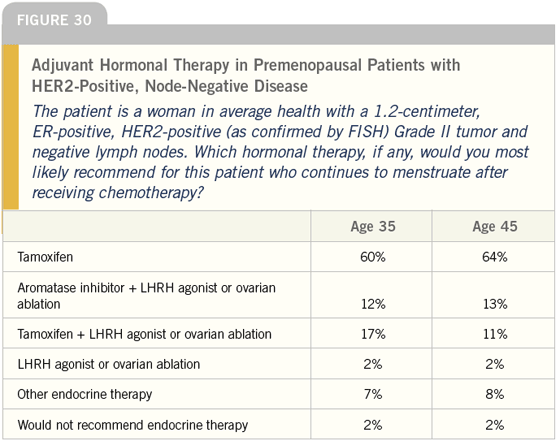

This makes a lot of sense to me. If you have breast cancer that is being signaled by HER2, and the ER is sitting next to it in the plasma membrane occupied by estrogen in premenopausal women, you can imagine that some crosstalk may occur between the ER (which can hetero- or homodimerize when it is occupied by the ligand) and the HER2.
If you then introduce tamoxifen into that setting, the drug will compete with estrogen for that estrogen receptor. It may not signal quite as strongly or be as good as removing the estrogen altogether like an aromatase inhibitor, but tamoxifen still may be of benefit in premenopausal women with ER-positive, HER2-positive disease. It is interesting and I think we need to analyze these studies carefully and separate the data from pre- and postmenopausal women.
Neil, for a premenopausal woman at low risk with a small, ER-positive, HER2- positive tumor with no positive nodes, I would treat her with tamoxifen. If she is at higher risk, has positive nodes or a T2-N0 tumor and has received chemotherapy and is still premenopausal, my first choice would be oophorectomy and an aromatase inhibitor. If it is not feasible for her to undergo an oophorectomy because she has not had children or completed childbearing, I will treat her with goserelin and anastrozole, but I have rarely done this.
DR LOVE: And you, Bob?
DR CARLSON: I tend to use tamoxifen in premenopausal women with ERpositive, HER2-positive disease, as the majority of physicians do. I think that the argument for ovarian suppression or ablation plus the addition of an aromatase inhibitor in this cohort of women is pretty compelling given the data that suggests aromatase inhibitors may be more effective in HER2-overexpressed breast cancer. However, I think we have to be cautious before we jump to that conclusion.
Breast tumors presenting during the premenopausal state may, in fact, respond quite differently to a hormonal intervention than breast tumors that appear in postmenopausal women; therefore, I don’t think we know whether the data suggesting the superiority of the aromatase inhibitors in HER2-overexpressing breast cancer is applicable to premenopausal women.
In the postmenopausal setting, physicians seem to be shifting away from tamoxifen toward the aromatase inhibitors, and shifting away from anastrozole toward letrozole. Presumably that trend is a result of Matt Ellis’ study evaluating tamoxifen versus letrozole. In that study, women with HER2- positive breast cancer appeared to have a superior response rate with letrozole.
This is a situation in which physicians may be making a drug-based conclusion that outweighs what I would call a disease-state conclusion. In HER2- overexpressed breast cancer, we do not have as much data for anastrozole as we do for letrozole; however, because their mechanisms of action are essentially identical, my expectation is that we are going to see identical outcomes from the two drugs.
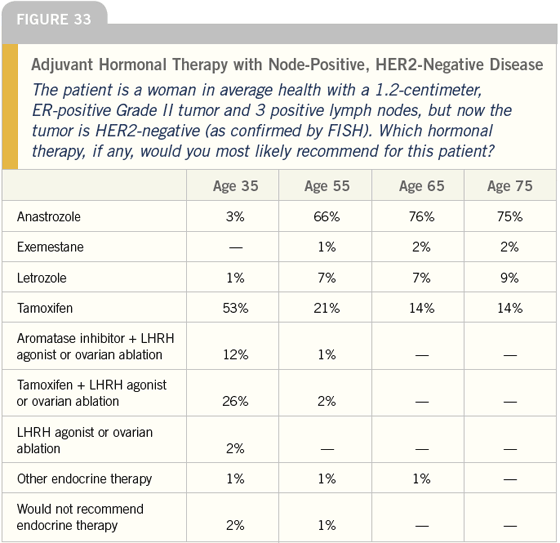
DR LOVE: Moving on to the next case, I think it is pretty interesting that 40 percent of the doctors we surveyed would use an LHRH agonist off study in the adjuvant setting for premenopausal women with positive nodes. Bob, what do you think about that (Figures 32 and 33)?
DR CARLSON: That number seems much higher than I would have expected. I think data exist to justify that approach and to argue against it. That seems to be a much higher frequency than I see in my community.
One of the difficulties in using ovarian ablation in this population is that you can’t take it back. It is such a huge shift for the woman — not only physiologically, but also psychologically — that I find it a very difficult step to take, especially with the uncertainty of all the data.
Ovarian suppression also requires monthly injections and substantial increased expense, and I believe that this is one of those situations in which an oncologist’s preconceived notions strongly influence the patient’s decisionmaking process.
DR LOVE: I take it that in this situation you are generally using tamoxifen in the younger woman and anastrozole in the older woman?
DR CARLSON: That’s correct.
DR LOVE: Joyce, from our discussions, I assume you would use anastrozole or an aromatase inhibitor for endocrine therapy in these patients.
DR O’SHAUGHNESSY: Yes, definitely.
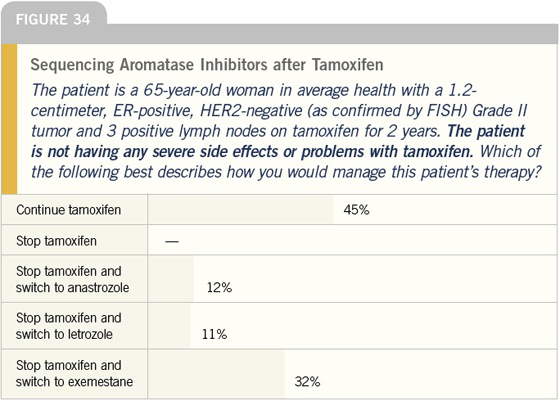
DR LOVE: Let’s discuss the issue of sequencing hormonal therapy. If the patient described here (Figure 34), who is not having any difficulty tolerating tamoxifen, came to you for a second opinion, what would you recommend?
DR O’SHAUGHNESSY: I discuss switching to an aromatase inhibitor with all my patients. Even if a patient’s systemic risk is incredibly low, the risk reduction for a new primary lesion is improved by switching to an aromatase inhibitor. For a patient who has taken adjuvant tamoxifen for two years, I use exemestane. For the patient who has taken tamoxifen for five years, or close to that, I use letrozole.
I am doing this pretty much across the board. I believe switching women from tamoxifen to exemestane has a huge upside and almost no downside. It is safer and allows us to avoid that low incidence of endometrial cancer. Switching is also efficacious.
Even for women who are at very low risk, their risk for second primary breast lesions is reduced by switching to an aromatase inhibitor, so I strongly favor it and I do it routinely.
DR LOVE: Do you do it at any time during the first five years?
DR O’SHAUGHNESSY: Every time I see a patient who is on tamoxifen, I evaluate whether I should switch her at that point or wait until the two- to three-year window. Rightly or wrongly, I think it boils down to which patient is going to have a late relapse and which one is going to have an early relapse.
My best guess is that it may have to do with grade and proliferation. If I have a patient with a strongly ER/PR-positive, Grade I-II tumor with a low proliferative rate, I believe she may be more at risk for a late relapse, so I tend to give that woman three years of tamoxifen and tell her to count on five years of an aromatase inhibitor.
However, when I see a patient on tamoxifen who has ER-positive, PR-negative, Grade II disease with a higher proliferative rate and more aggressive biology, I tend to switch sooner rather than later. I don’t necessarily wait two years. Most of these women start on an aromatase inhibitor from the beginning anyhow, so it is becoming a moot point, but if she were on tamoxifen for whatever reason, I would switch her as soon as I could.
DR LOVE: Which aromatase inhibitor would you use in a patient who has been on adjuvant tamoxifen for only six months?
DR O’SHAUGHNESSY: If I didn’t agree with the tamoxifen and if I wanted her on an aromatase inhibitor, I’d probably use anastrozole.
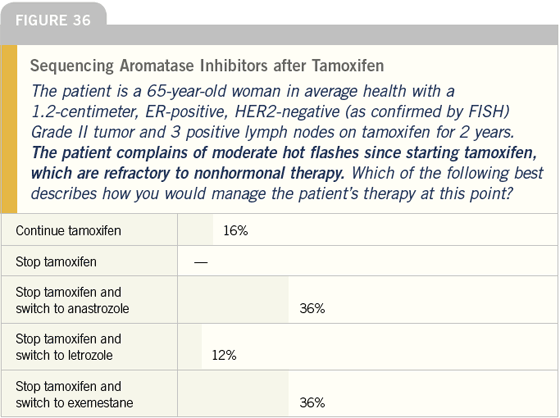
DR LOVE: Bob, how do you think the physicians in our survey did in managing this patient, and how do you approach this type of situation in your practice?
DR CARLSON: The numbers are certainly consistent with what I see in my community, but I think that they are going to change rapidly. In another year or two, I bet you will find 80 percent switching to an aromatase inhibitor.
In my practice, starting at two to three years, I begin to talk with women about the option of switching from tamoxifen to an aromatase inhibitor. I typically recommend a switch to exemestane, although I also talk about anastrozole because those are the two agents that we have data for in this specific situation.
I tell them that if they decline the switch at this point in time, when we reach the five-year time point we’ll have the discussion again, based on the extended adjuvant endocrine therapy data we have from the MA17 trial. To date, of women to whom I offer a switch, the vast majority are switching.
DR LOVE: What do you do if a woman has been on tamoxifen for six months or a year?
DR CARLSON: I recommend that she continue tamoxifen until the two- to three-year time point. We don’t fully understand whether the larger proportional risk reductions that we see with switching to the aromatase inhibitor compared with an aromatase inhibitor or tamoxifen up front, are related to selection of patients who do not have a recurrence and, therefore, are more likely to have hormone-responsive disease.
We also do not know if tamoxifen is somehow interacting with the biology of the tumor and changing it in such a way that it is more sensitive to the profound estrogen deprivation seen with an aromatase inhibitor. Until we sort these issues out, I think we should try to model our clinical decisions as closely to the clinical trials as we can.
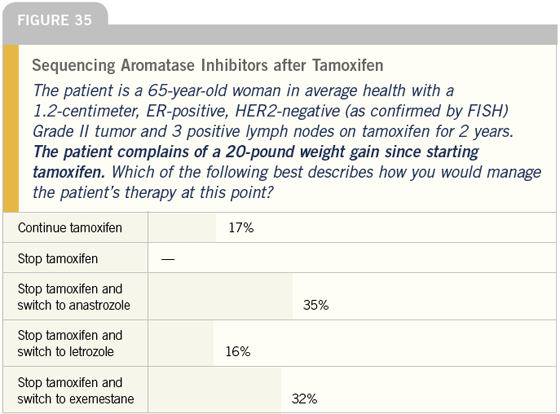
DR LOVE: It’s interesting that we see shifts toward aromatase inhibitors in women with excessive weight gain and hot flashes. What do you think about that (Figures 35 and 36)?
DR CARLSON: With regard to weight gain, I think this is a great example of people thinking with their biases as opposed to thinking with science. Randomized trials consistently show that tamoxifen does not cause weight gain, at least in comparison with placebo, and therefore switching to an aromatase inhibitor in this circumstance would be wishful thinking.
Women certainly have hot flashes on aromatase inhibitors, but I think they occur less often and are less intense than they are with tamoxifen. However, I tend to ignore hot flashes as a reason to switch or not switch from tamoxifen.
DR LOVE: Joyce, how does your practice compare with these responses (Figure 37)?
DR O’SHAUGHNESSY: I estimate that 50 percent of my patients on tamoxifen receive treatment for vasomotor symptoms, which is much higher than the responses you received. I consider an intervention when the patient experiences significant sleep disturbances. I find that women who are well rested can put up with hot flashes during the day, but if the therapy is causing symptoms such as night sweats, to the point of interrupting their sleep, the patient becomes exhausted and less able to tolerate these side effects. I treat these problems with trazodone, escitalopram oxalate or venlafaxine.
DR LOVE: And what is your experience with weight gain and tamoxifen (Figure 38)?
DR O’SHAUGHNESSY: I believe tamoxifen causes weight gain. The randomized data from NSABP-P-1 and even NSABP-B-14 showed that all the patients gained weight, whether they received the placebo or tamoxifen. We know that women after menopause gain weight every year, even if they eat and exercise exactly the same, because their metabolism slows. I believe tamoxifen exacerbates that in a subset of patients, despite the randomized data, and in general it seems to affect the patients who already have a weight problem.
DR LOVE: Do you discuss the issue of weight gain when you recommend tamoxifen therapy to these patients?
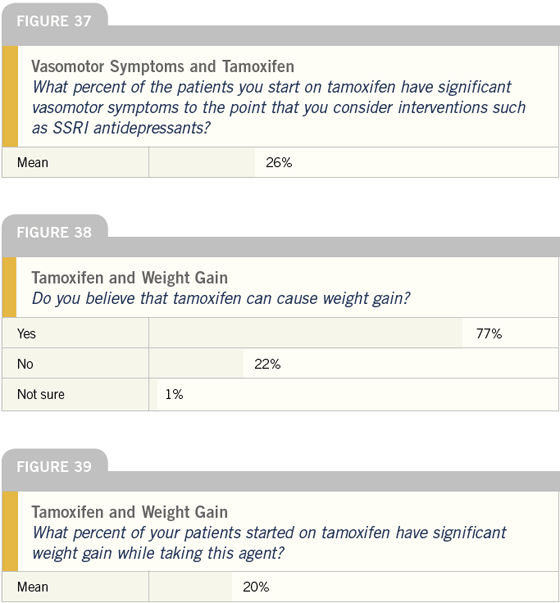
DR O’SHAUGHNESSY: I don’t bring it up, but the patients always do.
DR LOVE: What percent of patients in your practice have this problem (Figure 39)?
DR O’SHAUGHNESSY: During and after breast cancer treatment, whether the therapy is hormonal, chemotherapy or both, 80 percent of my patients gain weight. It’s a huge issue and it needs to be investigated. Also, I believe another 20 percent of patients gain weight if they are on tamoxifen.
DR LOVE: Moving on to the next case (Figure 40), how do you manage the patient who has completed five years of tamoxifen?
DR O’SHAUGHNESSY: After five years of tamoxifen, the only patients for whom I don’t recommend switching to letrozole are the older, postmenopausal women with low-risk disease — tiny lesions, node-negative — for whom I’m less concerned about the systemic risk for relapse. While patients over the age of 65 are still at risk for new primary lesions, they have had the benefit of five years of tamoxifen, so I don’t ask them to take more drugs.
DR LOVE: How do you decide on therapy for a patient who has been off adjuvant tamoxifen for one to three years?
DR O’SHAUGHNESSY: I treat them the same as the patient who has just completed tamoxifen. If I think they might benefit from a risk-reduction standpoint, that’s enough of a reason for me to consider further therapy.
DR LOVE: What would be your cutoff?
DR O’SHAUGHNESSY: It depends on the patient’s risk status. In a low-risk patient, I think five years would be my cut-off, but in a patient at high risk with multiple positive nodes, I probably have no time limit.
DR LOVE: Bob, what do you recommend to similar patients in these three situations?
DR CARLSON: I’m consistent with what these statistics show. After 5 years, I use letrozole as the preferred agent because that is where we have data.
About a year after stopping tamoxifen, I tend to not encourage crossing over to an aromatase inhibitor.
As more time passes after discontinuing tamoxifen, the further you get from the selection criteria used to justify the subsequent aromatase inhibitor in the clinical trials. I am not surprised that we see a drop in the frequency of aromatase inhibitor use.
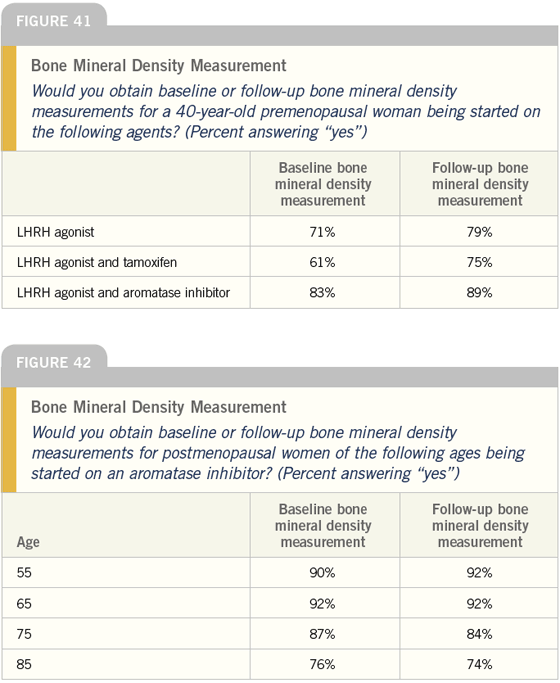
DR LOVE: Joyce, do you perform bone mineral density studies in these scenarios and in women of these varying ages (Figures 41 and 42)?
DR O’SHAUGHNESSY: Yes. I obtain baseline and follow-up bone mineral densities in patients receiving LHRH agonists, tamoxifen and aromatase inhibitors.
DR LOVE: Have you incorporated bone health into your care of breast cancer patients in general, or just those receiving endocrine intervention?
DR O’SHAUGHNESSY: If their primary physician is following their bone density, then I don’t, but if I have them on hormonal therapy then I feel obligated to do so.
DR LOVE: It appears from these responses that physicians are less likely to assess bone density in patients on tamoxifen. Do you agree with that practice?
DR O’SHAUGHNESSY: I don’t evaluate the bone as frequently in patients on tamoxifen; however, if the patient is also on an LHRH agonist, then I always assess their bone density. It’s been shown that this combination can cause bone loss and some postmenopausal women lose bone on just tamoxifen. Also, patients are generally not on tamoxifen for long and it’s important to have a baseline in case we switch them to an aromatase inhibitor later.
DR LOVE: With regard to the ER/PR status in patients with DCIS, what’s your practice and what do you think about these numbers (Figure 43)?
DR O’SHAUGHNESSY: We began assessing patients’ ER and PR status approximately four or five years ago and now we do it routinely. When Craig Allred presented his tamoxifen data from NSABP-B-24 at the 2002 San Antonio Breast Cancer Symposium, it solidified our practice. Prior to this data, we didn’t always insist on establishing the ER/PR status in patients we saw for second opinions, and we put them all on tamoxifen.
However, as we saw those women in follow-up, we checked their ER/PR status and I’ve since stopped tamoxifen in patients whose tumors were negative, even if they had been on it for a couple of years. Now I use tamoxifen only in patients with DCIS who are ER- and/ or PR-positive and DCIS clinical trials like NSABP-B-35, in which patients are randomly assigned to tamoxifen versus anastrozole, restrict eligibility to patients with receptor-positive disease.
I reviewed this issue recently as I wrote a chapter on systemic therapy for DCIS for Martine Piccart’s book, which examines molecularly targeted breast cancer treatment. In the data from NSABP-B-24, little evidence indicates that patients with truly ER/PR-negative disease derived benefit from tamoxifen.
While relatively little data exists, based on what we do have, I don’t believe we should expect tamoxifen to benefit patients with ER/PR-negative DCIS — in preventing in-breast recurrences or reducing the risk of a contralateral breast cancer — just as it doesn’t benefit patients with ER/PR-negative breast cancer. Given the risks associated with tamoxifen, I’d like to see more clinicians considering the ER and PR status when deciding whether to use tamoxifen in patients with DCIS.
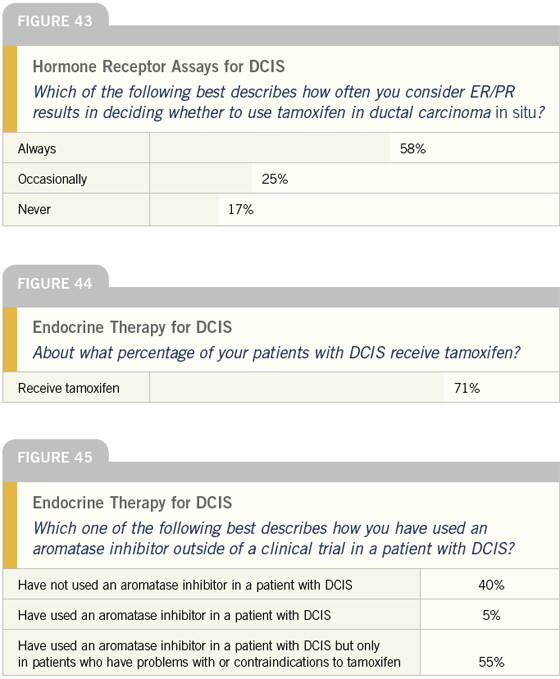
DR LOVE: In your practice, what percent of patients with DCIS actually receive tamoxifen (Figure 44)?
DR O’SHAUGHNESSY: I would estimate that 75 to 80 percent of my patients with DCIS receive tamoxifen. If the disease was treated by lumpectomy and radiation therapy, I tend to use tamoxifen to prevent an in-breast recurrence and a new primary lesion.
In a woman over the age of 60 with ERpositive DCIS, who has undergone a mastectomy, I would estimate the risk of a new primary lesion or a contralateral occurrence to be in the neighborhood of 0.5 to 0.8 percent per year. In the next 20 to 25 years, she has approximately a 10 to 16 percent risk of developing a new second primary lesion. Tamoxifen reduces that risk by 50 percent; however, I don’t recommend tamoxifen for all of these patients. If the risk of a new primary lesion or DCIS is only 10 percent or less, I worry more about the risks of tamoxifen — thromboembolic risk, stroke, CVAs, DVTs — particularly in women over the age of 60.
I also consider the tissue background. In a patient who undergoes a mastectomy, we have a good deal of tissue to examine. If the background is relatively bland, I am less inclined to recommend tamoxifen to protect the contralateral breast.
On the other hand, if a good deal of atypia or LCIS is present, for example, I worry more about the contralateral breast. Likewise, if a patient has a strong family history of breast cancer, I’m more inclined to worry about a contralateral new primary lesion.
DR LOVE: How do you feel about the off-protocol use of aromatase inhibitors for DCIS in patients who can’t take tamoxifen (Figure 45)?
DR O’SHAUGHNESSY: I have used them sparingly in patients at very high risk, such as those with multifocal DCIS, but only when tamoxifen is absolutely contraindicated. We have very little data on this issue.
DR LOVE: If clinical trials like NSABPB- 35 show the efficacy of aromatase inhibitors to be equal to, but not greater than, tamoxifen, would you switch your patients to these agents given their sideeffect profiles?
DR O’SHAUGHNESSY: Definitely, because in the risk-reduction setting, we don’t want patients to experience any unnecessary side effects.
Select publications
|

