| |
Adjuvant Systemic Therapy for Colon Cancer |
Colorectal Cancer Update 2005 (2) |
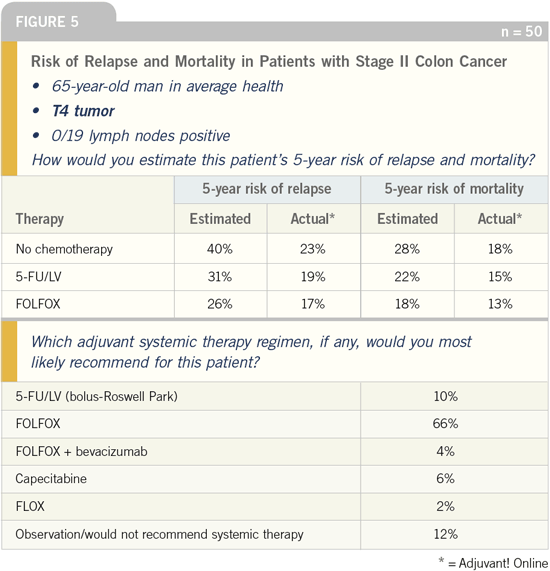
DR JOHN MARSHALL: It is interesting
that we tend to back away from adjuvant
therapy in patients who have a lower risk,
when it may be more appropriate to do
exactly the opposite. I think that those
are the patients with whom we should
be the most aggressive. In the Stage II
subset analysis of the MOSAIC study,
the patients who received FOLFOX
had a three-year disease-free survival of
87 percent. To my knowledge, that’s the
highest number ever reported for Stage
II patients.
In breast cancer we are accustomed
to utilizing adjuvant chemotherapy for
relatively small gains, meaning two to
four percent absolute gain. I believe we
should be equally aggressive when treating
patients with colon cancer, and we
should incorporate these adjuvant therapies
as often as possible. By adopting
these new therapies, we’re going to cure
more patients of this disease.
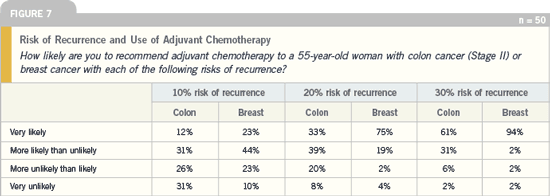
DR LOVE: What are your thoughts on
these patterns of data related to the
risk of recurrence and the use of adjuvant
chemotherapy in breast and colon
cancer (Figure 7)?
DR AXEL GROTHEY: This is very interesting
and reflects what we see in practice,
particularly in terms of the 10 percent
risk of recurrence. When the categories
of very likely and more likely than unlikely are combined, more than 65 percent of
the breast cancer patients would receive
chemotherapy, while approximately 40
percent of the colon cancer patients would
receive chemotherapy. That is a significant
difference, and this is relevant.
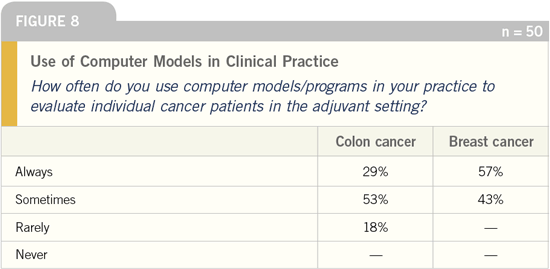
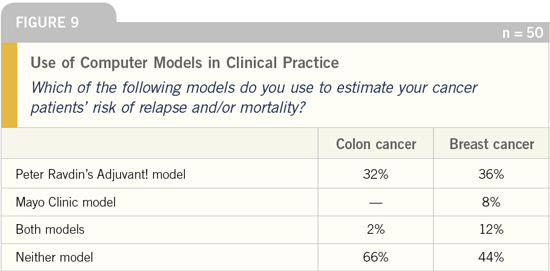
DR LOVE: In a recent colorectal cancer
think tank, Peter Ravdin mentioned that
the number of people utilizing the colon
cancer Adjuvant! model is about one
tenth the number who utilize the breast
cancer Adjuvant! model.
What was your take in this survey
on the number of oncologists utilizing
the Adjuvant! model for both breast and
colon cancer risk estimates?
DR GROTHEY: I thought that it was very
interesting that the oncologists appear
to be using the models so much more in
breast cancer. I believe that the oncologists’
predictions for recurrence and
mortality in the adjuvant setting for
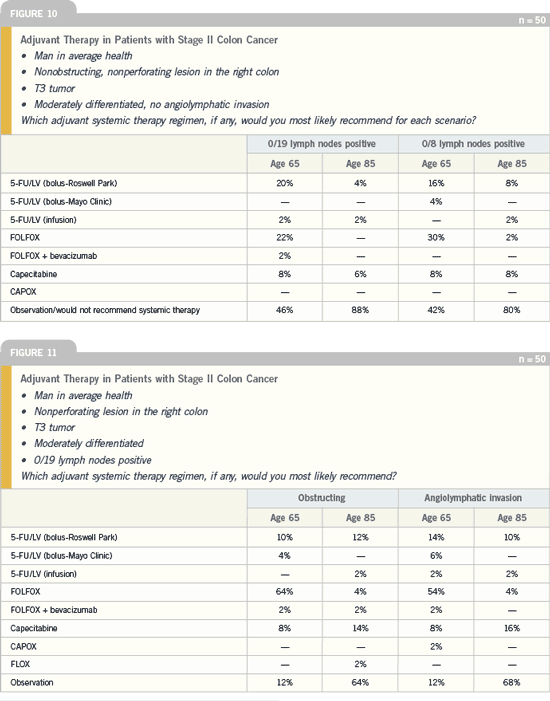
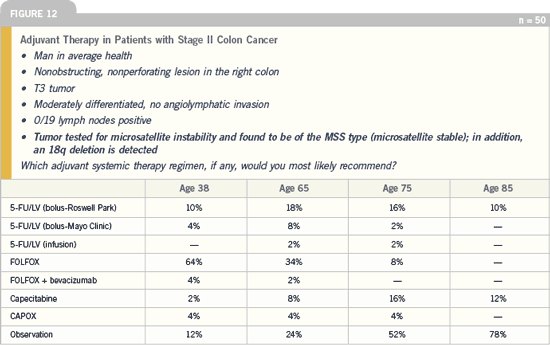
Stage II and Stage III patients are also quite accurate (Figures 10-12).
Click here to see image
DR LOVE: According to the survey data,
adverse risk factors such as angiolymphatic
invasion, obstruction and microsatellite
instability have significant impacts
on whether or not doctors choose to
recommend chemotherapy (Figures 10-
12); do you support this observation?
DR GROTHEY: Yes, if an adverse risk
factor can be identified, it clearly shifts
toward the recommendation of chemotherapy.
That is exactly what has
happened with this data — risk factors
were identified and chemotherapy was
recommended.
I was surprised that the molecular
risk factor — the microsatellite-stable
patient with 18q deletion — actually
shifted practice as much as some of the
clinical factors.
Furthermore, I was a little bit concerned
about the magnitude of difference
in the shift toward treating for an
obstructing tumor compared to treatment
for a tumor that had an inadequate
sample size of lymph nodes — zero of
eight lymph nodes.
The awareness of the importance of
lymph-node dissection, which is a very,
very strong prognostic factor, is not as
large as it should be. That factor is as
important as obstruction or other clinical
risk factors.
Click here to see image
Click here to see image
Colorectal Cancer MTP September 2005 |
DR LOVE: In general, for Stage II
disease, which regimen do you generally
utilize when you treat a patient in
their sixties?
DR GEORGE FISHER: For patients
in good health, I would have no real
concerns about the safety of administering
chemotherapy. It is a question of
how much discomfort that person would
be willing to tolerate for six months.
In a patient with high-risk disease
who wanted to receive a regimen that
offered the highest absolute benefit, then
that would be a FOLFOX regimen.
And if that person was shy of doctors’
visits, IVs, needles, 48-hour infusions, or
had catheter contraindications, I believe
that CAPOX is certainly a suitable
alternative.
Colorectal Cancer Update 2005 (4) |
DR LOVE: What are your thoughts about
adjuvant chemotherapy for patients with
Stage II colon cancer?
DR ROBERT DIASIO: Typically, patients
with no evidence of lymph node involvement,
no matter how deeply the tumor
appears to extend, do not receive chemotherapy
for Stage II disease. However,
increasing data suggest that some patients
with penetration of the intestinal wall,
who would not have been treated in the
past, may benefit from chemotherapy.
The ASCO committee published an
aggressive position paper stating that perhaps
Stage II patients should be offered
adjuvant therapy. While we don’t have
any convincing objective data to validate
the use of adjuvant therapy in Stage II
disease, subsets within that population
may benefit. The ultimate proof of the
benefit in such patients will come from
ongoing adjuvant studies.
One reason it may be difficult to demonstrate
a benefit from adjuvant therapy
in Stage II disease is that fewer events
occur. However, the MOSAIC trial and
some of the earlier Intergroup studies
have suggested certain patients can
benefit from chemotherapy.
Colorectal Cancer Update 2005 (3) |
DR LOVE: In general, what is your
approach to adjuvant therapy in patients
with high-risk Stage II colon cancer?
DR AIMERY de GRAMONT: I would
certainly offer adjuvant FOLFOX to
patients with Stage III or high-risk
Stage II disease.
In patients with a very good prognosis,
the potential risks and benefits of an
adjuvant regimen must be weighed in a
discussion that should occur between
the patient and physician.
We presented data from the patients
with Stage II disease in the MOSAIC
adjuvant trial. In an analysis of the
patients with high-risk Stage II disease
(eg, T4, bowel obstruction, tumor perforation,
venous invasion or fewer than 10
lymph nodes analyzed), the difference in
disease-free survival in favor of FOLFOX
was over five percent.
In patients with high-risk Stage II
disease, adjuvant FOLFOX should
be considered.
Click here to see image
Colorectal Cancer Update 2005 (3) |
DR PAULO HOFF: For those patients who
present with Stage II disease, the decision
about the use of adjuvant chemotherapy
is complicated. Obviously, we
have to discuss the potential benefits
and toxicities of chemotherapy. I tend
to suggest adjuvant chemotherapy more
strongly if their disease has a high-risk
feature (eg, obstruction, perforation or
lymphovascular invasion).
Once I explain all the options, even
some patients in whom I would prefer to
use FOLFOX surprisingly ask to receive
capecitabine. They feel attracted to the
oral agent. Also, for patients with severe
comorbid conditions or the very frail
elderly patients, I tend to use adjuvant
capecitabine instead of FOLFOX.
Colorectal Cancer Update 2005 (4) |
DR LOVE: Do you think that capecitabine
can be utilized in the adjuvant setting as
a substitute for 5-FU?
DR PHILIP PHILIP: In situations in
which a single-agent fluoropyrimidine is
being used or contemplated, capecitabine
should be used. I don’t believe at this
time that, if given the option, a patient
will opt for intravenous treatment unless
an issue arises regarding who will pay for
the capecitabine. Capecitabine should
be the drug of choice for patients who
will receive a single-agent fluoropyrimidine
because it’s easier to administer
and doesn’t interfere much with the
patient’s daily routine. It has side effects,
and we have to pay attention to them.
But overall, it’s a treatment that patients
will prefer.
In which patients should we use
single-agent therapy? In patients with
Stage III disease, the data on adjuvant
FOLFOX have completely transformed
my practice. I use FOLFOX in patients
with Stage III disease, except in those
who refuse the combination, cannot take
a neurotoxic drug or are too old for such
a combination. Those patients who don’t
receive adjuvant FOLFOX receive single-agent
capecitabine. The next question
becomes, Can we combine capecitabine
with oxaliplatin? Adjuvant CAPOX is
still experimental, and it should be used
as part of a clinical trial. We still have
to wait for the head-to-head comparison
with FOLFOX.
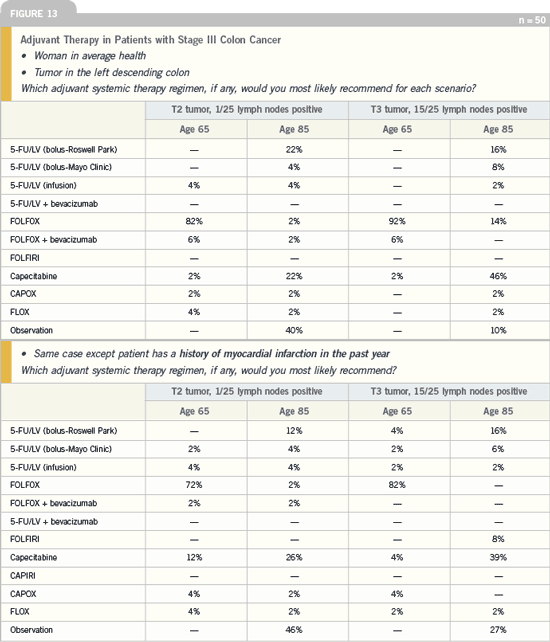
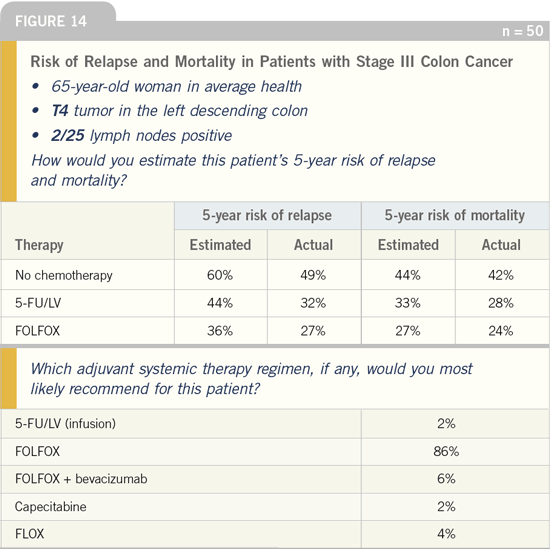
DR LOVE: In general, what adjuvant
chemotherapy would you be most likely
to recommend to a patient with Stage III
colon cancer who had a T2 tumor and
one of 25 positive lymph nodes?
DR GROTHEY: I would recommend
FOLFOX for a 38-, 65- or 75-year-old
patient. In an 85-year-old patient I would
more than likely utilize capecitabine.
DR LOVE: The survey shows that in
the adjuvant setting for the 85-yearold
patient with lower-risk disease, the
frequency of using 5-FU monotherapy is
approximately the same as the amount of
capecitabine being given.
DR GROTHEY: Yes, and this is happening
based on extensive experience with 5-FU
regimens and the fact that the dosing
of capecitabine has not been completely
established in the United States. There
is concern about the toxicity associated
with capecitabine. However, there is clear
advantage with capecitabine in terms
of convenience. There’s no doubt about
that. I would prefer to see more patients
on capecitabine than on bolus 5-FU.
DR LOVE: What therapy should be
recommended to a Stage III patient who
is concerned about oxaliplatin-associated
neuropathy and would prefer not to take
an oxaliplatin-containing regimen?
DR GROTHEY: In that situation
capecitabine should be recommended.
However, most patients can tolerate some
oxaliplatin. Cumulative toxicity does
not occur within the first three or four
months. Whatever regimen you choose
to combine with oxaliplatin — whether
you utilize FLOX or FOLFOX — there
is evidence that a little bit of oxaliplatin
is better that none. We assume that the
same holds true for combining oxaliplatin
with capecitabine-based regimens.
Colorectal Cancer Update 2005 (5) |
DR LOVE: What is your opinion of the
NSABP-C-07 trial comparing Roswell
Park 5-FU versus FLOX?
DR GROTHEY: The results from
NSABP-C-07 were more positive than
most experts expected. NSABP-C-07
randomly assigned patients with Stage II
or III colon cancer to receive the Roswell
Park regimen of 5-FU/leucovorin
(three cycles of an eight-week regimen)
with or without oxaliplatin 85 mg/m2
administered at weeks one, three and
five (FLOX).
Compared to the FOLFOX4 regimen
used in the MOSAIC adjuvant trial,
the FLOX regimen in NSABP-C-07
had the same duration of therapy but a
lower cumulative dose of oxaliplatin (765
mg/m2 versus 1,020 mg/m2). Although
the dose intensity of oxaliplatin was
lower and a bolus 5-FU regimen was
used as the backbone for the FLOX regimen,
they found an increase in the three-year
disease-free survival that was almost
identical to that in the MOSAIC trial:
about a five percent absolute increase.
This suggests that the addition of
oxaliplatin to any 5-FU-based regimen
is of benefit in the adjuvant setting.
Secondly, it shows we probably have two
alternatives to choose from: FOLFOX
or FLOX.
Colorectal Cancer Update 2005 (3) |
DR LOVE: How has the X-ACT trial
impacted your utilization of capecitabine
in the adjuvant setting?
DR MICHAEL O’CONNELL: The X-ACT
trial established the principle that oral
chemotherapy could be effective in the
adjuvant setting, compared to intravenous
chemotherapy. Capecitabine offers
the patient the advantage of not requiring
IV injections. The dosage level that
was used is a bit higher than most oncologists
in the United States have been
able to administer to their patients, and
it raises some interesting questions about
possible pharmacogenetic differences
between the populations in Europe and
the United States.
I believe the data are very compelling
and suggest that there might be an
advantage for capecitabine over the Mayo
Clinic method of administering 5-FU
and leucovorin in the primary endpoint
of disease-free survival, which practically
reached statistical significance in favor
of the capecitabine. The primary goal of
the study was to demonstrate noninferiority.
They certainly accomplished that.
I now believe that in clinical practice,
for a patient in whom fluoropyrimidine
therapy is considered appropriate,
capecitabine is a viable option.
Colorectal Cancer Update 2004 (5) |
DR HOCHSTER: The X-ACT trial was a
comparison of adjuvant capecitabine to
the Mayo Clinic 5-FU regimen. We now
know adjuvant capecitabine is equal to
or perhaps slightly better than the Mayo
Clinic regimen. I think that’s a very
important observation, and adjuvant
capecitabine is a reasonable option for a
well-educated patient who can be relied
upon to take pills on a regular basis.
This requires a highly motivated
patient who will call you or come in when
they start to develop diarrhea, handfoot
syndrome or any of the toxicities.
I don’t have a hesitation to use adjuvant
capecitabine, based on the clinical data at
this point in the adjuvant setting.
Colorectal Cancer Update 2005 (3) |
DR LOVE: In general, what is your treatment
approach in the adjuvant setting for
a patient with Stage III colon cancer?
DR LEONARD SALTZ: I’m pretty comfortable
with the MOSAIC data, so I generally
use FOLFOX in the adjuvant setting
for patients with Stage III disease. When
I have a patient who is particularly dependent
on their fine-motor skills, I discuss
with them whether we want to include
oxaliplatin in their treatment because the
neurotoxicity might compromise their
quality of life. If I’m concerned about a
patient’s ability to tolerate combination
chemotherapy, I might consider using
one of several schedules of 5-FU/leucovorin
or capecitabine.
Colorectal Cancer Update 2005 (1) |
DR CHRIS TWELVES: In the MOSAIC
trial, the addition of oxaliplatin resulted
in a significant reduction in the risk of
recurrence in the adjuvant setting.
I believe the MOSAIC data are the
new gold standard. Only time will tell
what that means for individual patients.
A gold standard doesn’t necessarily mean
the therapy applies to all patients. There
are toxicities related to oxaliplatin, such
as myelosuppression and neurotoxicity,
and I don’t believe oxaliplatin-based
adjuvant therapy will replace single-agent
treatment across the board.
I anticipate a rapid move towards
oxaliplatin-based treatments, especially
in the younger, fitter and higher-risk
patients. However, I believe a single-agent
fluoropyrimidine will still be an appropriate
option for a substantial proportion
of older, more frail patients or patients at
lower risk of disease recurrence.
Colorectal Cancer Update 2005 (1) |
DR LOVE: Can you discuss the ongoing
NSABP trial investigating the addition
of bevacizumab to FOLFOX in the adjuvant
setting?
DR NORMAN WOLMARK: The NSABPC-08 trial opened in October 2004. The
trial design is simple and straightforward
— modified FOLFOX-6 with or
without one year of bevacizumab. The
eligibility criteria include patients with
Dukes’ B or C colon cancer.
Originally, we wanted to make this
trial as broad-based as possible and
include FLOX (bolus
5-FU/leucovorin/oxaliplatin). The FDA didn’t particularly
embrace that idea; their response was
justified because we didn’t have data from
NSABP-C-07. In view of the MOSAIC
adjuvant trial data with a FOLFOX regimen,
I think a FOLFOX-inspired regimen
is reasonable. So we eliminated the
possibility of having FLOX as a control
arm. Also, we were thinking of including
a capecitabine/oxaliplatin (CAPOX) arm, but the sample size would have been
much greater.
We really wanted to address a pivotal
question — whether the benefits
associated with bevacizumab as first-line
therapy for metastatic colorectal cancer
can be translated to the adjuvant setting.
Once we came to grips with that as our
unequivocal principal aim, the trial was
structured to address it.
The sample size is manageable at
about 2,600 patients. Theoretically, we
hope bevacizumab will be more effective
in the adjuvant setting. We hope
the prolongation in time to progression
seen in patients with advanced disease,
if translated to the adjuvant setting, will
result in lives saved.
Colorectal Cancer Update 2005 (2) |
DR ALAN VENOOK: In my opinion, the
flaw in treating patients with Stage II
disease in the NSABP C-08 trial evaluating
FOLFOX with and without bevacizumab
is the accumulating evidence
that a subset of patients with Stage II
disease should not be subjected to the
risk of chemotherapy.
ECOG is addressing that issue with
a clever trial design that risk stratifies
patients with node-negative disease.
This stratification is based on the molecular
features of the tumors.
For example, patients who have
normal 18q are observed without
therapy, based on retrospective data
from a number of studies suggesting
that those patients do well, while patients
in the study who have deletion of 18q
are randomly assigned to chemotherapy.
A relative risk reduction occurs with
colorectal cancer chemotherapy, so the
issue lies in identifying the baseline risk.
FOLFOX causes neuropathy, so in a
patient with node-negative disease who
may have an 82 percent likelihood of
being alive and disease free five years
later, you have to balance the benefit
with the long-term consequence.
Colorectal Cancer Update 2005 (4) |
DR PHILIP: The specific question that
is being asked by NSABP-C-08 relates
to whether there is a benefit to adding
bevacizumab to FOLFOX. The duration
of therapy with bevacizumab is
also of interest in this study because it
continues after adjuvant chemotherapy
for another six months.
We also have to evaluate the toxicity
associated with this regimen because
of what we’ve seen with bevacizumab.
NSABP-C-08 is a good trial because the
best use of bevacizumab might be early
in the natural history of the disease.
This may be the way to go, but one of
the concerns with the regimen is, obviously,
toxicity. We’ll need to see what
happens.
Colorectal Cancer MTP September 2005 |
DR LOVE: Is there a role for irinotecan
in the adjuvant setting, for example, in a
patient with neuropathy or who cannot
tolerate oxaliplatin?
DR PETER ENZINGER: The PETACC
trial investigated infusional 5-FU/
leucovorin with or without irinotecan.
In that trial the primary endpoint was not statistically significant.
However, I believe that there is some
borderline benefit for using irinotecan
in the adjuvant setting. You could argue
that in a patient who has high-risk Stage
III disease but for some reason upon
receiving the first dose of FOLFOX has
an allergic reaction to oxaliplatin, that
may be a patient in whom you could use
the FOLFIRI regimen.
The ACCORD study specifically
looked at high-risk colon cancer patients
and did not identify a difference between
the patients who received irinotecan and
those who did not. That being said,
it was clear that investigators removed
their patients — or the patients removed
themselves — from the study, if they
were randomized to receive only 5-FU
and leucovorin. The bottom line, in my
mind, is that FOLFIRI may be an option
in a patient who wishes to receive aggressive
therapy and who cannot, for some
reason or another, tolerate oxaliplatin.
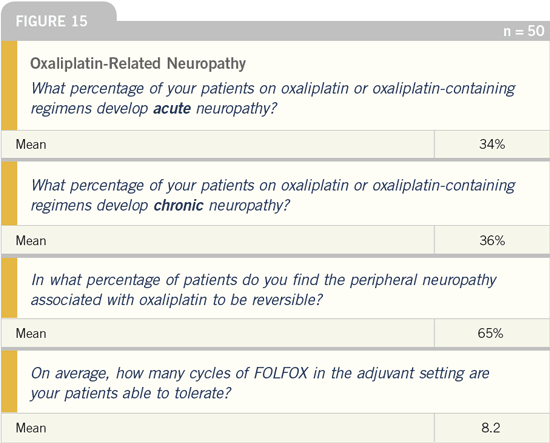
DR LOVE: In your experience, what
percent of patients on oxaliplatin-containing
regimens develop acute
neuropathy?
DR GROTHEY: Approximately 90 percent
of patients develop cold-induced symptoms.
The mean response to the question
regarding oxaliplatin-related neuropathy
(Figure 15) is considerably less than I
would have expected. Perhaps the physicians
are not aware of this — so this may
define an educational need.
When you ask a patient a subjective
question, such as whether they
have experienced side effects, it is a very
dynamic process. If you do not ask, the
patients may not forward the information.
However, when you ask a patient
directly, “So, did you have any side effects
from the treatment?” I would expect that
more than 70 percent of patients would
respond, “Yes, I experienced some nerve
problems.” This is completely underrepresented,
and it may be due to confusion
identifying acute and chronic
neuropathy — which is important for
clinical management.
DR LOVE: In terms of chronic neuropathy,
do you agree with the mean response
of 36 percent?
DR GROTHEY: This question is left open
in terms of severity. In the end, it’s
a matter of how you define chronic
neuropathy. I would say this is more likely what the physicians perceive. Almost
every patient experiences some form of
neuropathy, although it might not affect
activities of daily living. So perhaps a
better question would be, What percentage
of your patients develops chronic
neuropathy that affects the activities of
daily living?
DR LOVE: Do you agree with the survey
response that patients are able to tolerate
an average of eight cycles of an oxaliplatin-containing regimen?
DR GROTHEY: Yes.
Colorectal Cancer Update 2004 (4) |
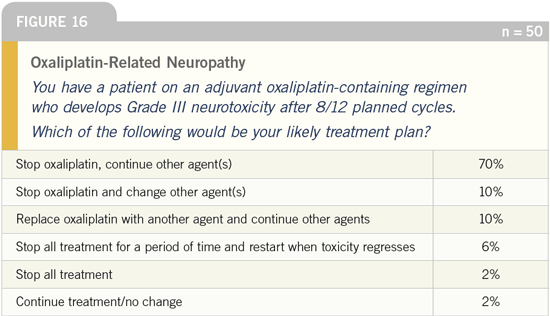
DR LOVE: Do you find that most patients
are able to tolerate oxaliplatin-related
neuropathy?
DR FISHER: Certainly the toxicity seems
tolerable. In the MOSAIC trial, about
18 percent of the participants had Grade
III neuropathy during or shortly after
the study. At one-year follow-up, that
decreased to one percent. Grade III
neuropathy is no fun, but patients have
been living with cisplatin neurotoxicity
for years. I think adjuvant FOLFOX is
finding believers, not only in academic
circles but also in the community.
In particular, it’s being used for young
patients with high-risk Stage III disease.
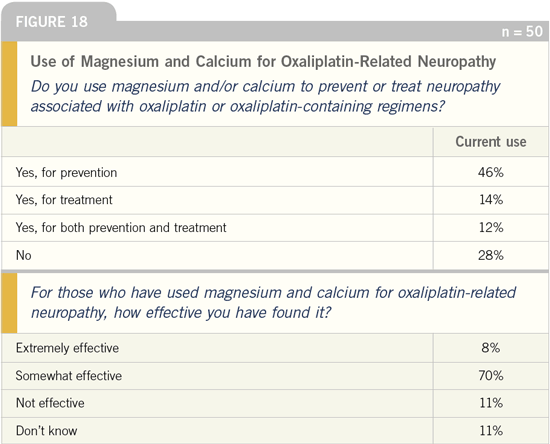
DR LOVE: Do you use calcium and
magnesium to prevent oxaliplatin-related
neuropathy?
DR GROTHEY: We utilize these agents
within a clinical trial. We currently have
a placebo-controlled trial — calcium/magnesium versus placebo in the adjuvant
setting — that we proposed to run
through the NCCTG. However, one of
the comments that we have received was
that physicians were reluctant to enroll
patients in the placebo arm because they
are using calcium/magnesium in their
clinical practice.
Seventy-eight percent of the physicians
surveyed who use magnesium and
calcium believe it is effective. This is
higher than I would have expected, but
on the other hand, physicians wouldn’t
use it if they didn’t see any effectiveness.
Colorectal Cancer Update 2005 (1) |
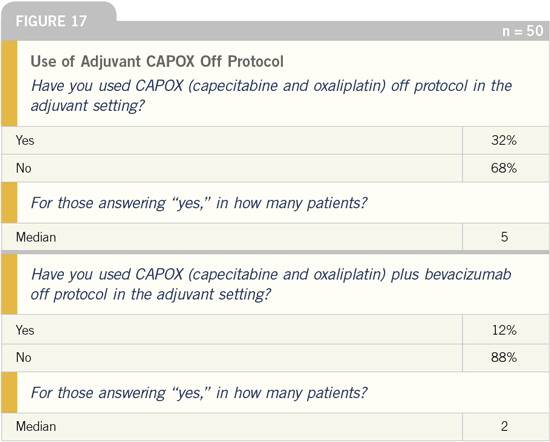
DR LOVE: What are your thoughts on
the role of CAPOX in the adjuvant and
metastatic settings?
DR TWELVES: As one who participates
in clinical trials, I prefer to wait for
evidence from randomized studies before
using new combinations off protocol in
the adjuvant and metastatic settings.
However, with CAPOX I’m torn because
everything we’ve seen to date from the
clinical trials suggests that 5-FU can be
substituted with capecitabine in these
clinical settings. In addition, I would
be very surprised if CAPOX doesn’t
emerge as being equivalent to the FOLFOX
regimen, alone or in combination
with bevacizumab. I do believe CAPOX,
off protocol, is a reasonable option at
this time.
Colorectal Cancer Update 2005 (3) |
DR HOFF: There is great interest, especially
in the community, in having an
oral chemotherapy-based regimen, and
the CAPOX regimen is very attractive
in that regard. Given the opportunity,
patients tend to choose oral agents. We
have the X-ACT adjuvant study showing
that capecitabine was equivalent and
had a hint of being better than bolus
5-FU/leucovorin. I think the data from
the Phase II CAPOX trials in the
advanced setting are intriguing enough
to say that it’s at least equivalent to
FOLFOX.
I wouldn’t recommend CAPOX
as my first option in the adjuvant setting,
because obviously we prefer to use
evidence-based medicine. However, I
would not necessarily find it incorrect
to use CAPOX in the adjuvant setting.
Scientifically, it makes sense.
Colorectal Cancer MTP September 2005 |
DR LOVE: Can you outline the design of
the international AVANT trial?
DR WOLFF: The AVANT trial investigates
adjuvant therapy for patients with
Stage III colon cancer. There are three
arms to that trial, and there are two
questions being asked. The first question
being addressed is, Are CAPOX
and FOLFOX equivalent? The second
question is, Does bevacizumab add to
adjuvant therapy?
Patients on the first arm will receive
FOLFOX alone. Patients on the second
arm will receive FOLFOX plus bevacizumab.
And patients in the third arm
will receive CAPOX plus bevacizumab.
However, there is not an arm directly
comparing CAPOX versus FOLFOX.
My belief is that bevacizumab will work
with both the CAPOX and FOLFOX.
What we will learn from this study
is whether or not bevacizumab adds
to standard adjuvant chemotherapy and
whether CAPOX and FOLFOX are
equivalent in terms of efficacy as adjuvant
chemotherapy.
This study is very nicely done. I do
not believe that the toxicity is going to be
unmanageable based on our experience
with these regimens in the past.
Select publications
|

