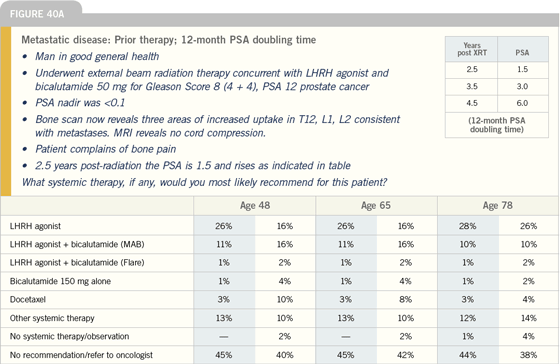
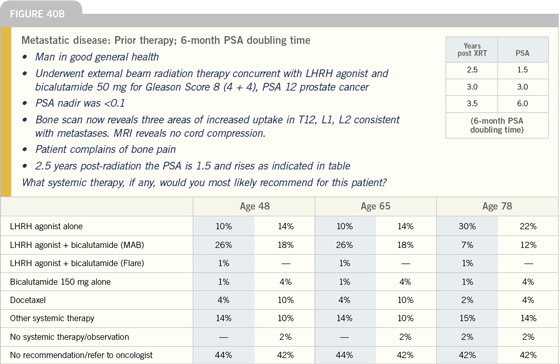
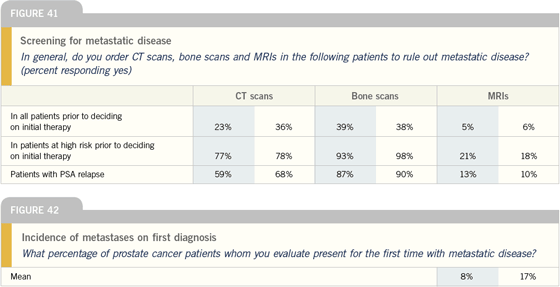
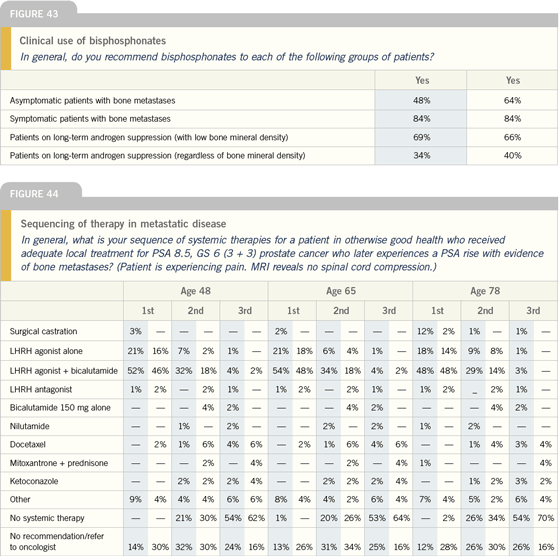
| |
Management of Metastatic Disease |

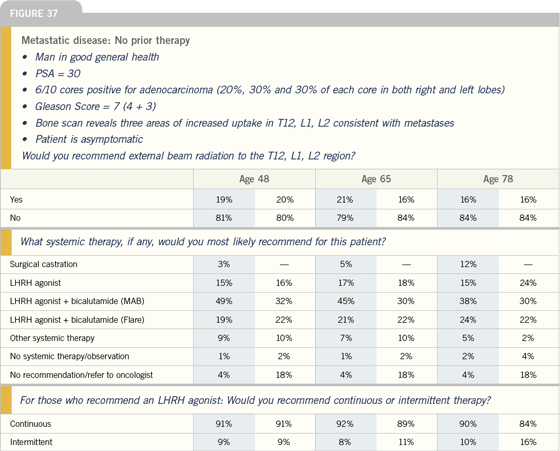
Click here to see image
Benefits of MAB with
bicalutamide in patients
with metastatic disease
| Prostate Cancer Update 2005 (3) |
DR KLOTZ: Our analysis integrated the
results from trials with a common treatment
arm in situations in which it’s
no longer feasible to conduct a placebo-
controlled trial. Dr Schellhammer
compared bicalutamide to flutamide
with goserelin or leuprolide and demonstrated
a 13 percent reduction in the
risk of death for the patients receiving
bicalutamide compared to those receiving
flutamide.
We integrated those data with the
results from the meta-analysis of the
Prostate Cancer Trialists’ Collaborative
Group, which demonstrated a significant
eight percent reduction in the risk
of death for patients treated with flutamide
plus castration compared to those
treated with castration alone. The flutamide/
castration arm can be cancelled
out, and you end up with a comparison
of bicalutamide plus castration to castration
alone even though they have never
been directly compared. This analysis
demonstrated a 20 percent reduction in
the risk of death for patients treated with
bicalutamide plus castration.
Use of maximal
androgen blockade
| Prostate Cancer Update 2005 (1) |
DR PETRYLAK: The survival data from the
SWOG studies — particularly SWOG-
8494, in which Dave Crawford was the
principal investigator — showed approximately
a three-month improvement in
survival in favor of combined blockade
compared to an LHRH agonist alone.
I use maximal androgen blockade.
Certainly, we’ve treated patients with
more aggressive therapy for less of a survival
benefit. I believe it can’t hurt. And
if it can’t hurt and has a possibility of improving survival, I will use the combined
blockade with bicalutamide, which
is the easiest drug for me to administer
and for the patient to receive.
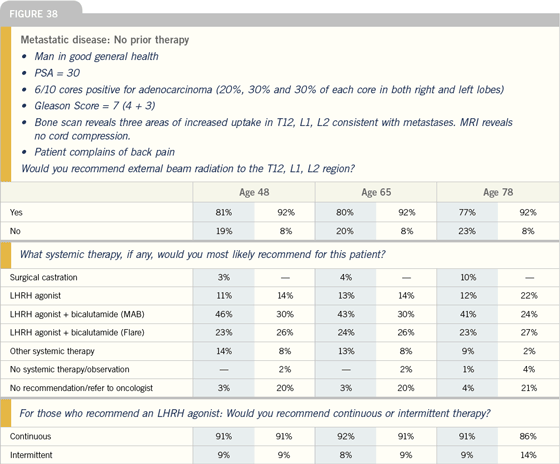
Click here to see image
Intermittent versus continuous
androgen deprivation
| Prostate Cancer Update 2005 (3) |
DR CRAWFORD: SWOG-S9346, which
has been ongoing for a number of years,
has accrued about 2,000 patients. Men
with newly diagnosed, untreated metastatic
disease receive combined androgen
ablation. At nine months, if their PSA
drops below 4 ng/mL, they are randomly
assigned to continuous or intermittent
therapy. With intermittent therapy, the
patient resumes hormonal therapy when
his PSA goes up to a predetermined level
— usually half of the baseline level or 10
ng/mL.
The whole idea is to provide a hormonal
therapy holiday to reduce toxicity
and costs. Integrated in that trial is
the use of bisphosphonates, particularly
zoledronic acid, to evaluate their effects
on bone disease.
We have enough data suggesting
that intermittent therapy is probably
not going to make the patient’s scenario
worse; at least that’s what has been
reported.
Whether it’s going to be better is
unknown. If the benefit is the same as
for continuous therapy, it’s a no-brainer
that intermittent therapy would be the
choice, since patients can have a drug
holiday with fewer side effects and less
expense.
Earlier integration of medical
oncologists in management
| Prostate Cancer Update 2005 (1) |
DR PETRYLAK: In the community, urologists
usually attempt a couple of hormonal
manipulations and then send their
patients to the oncologist. The optimal
time to start chemotherapy is a bit of an
art, and no FDA guidelines delineate the proper time to start chemotherapy.
Not all patients with hormone refractory
disease should start chemotherapy. I
believe patients should see an oncologist
initially, but they should never lose contact
with their urologist. The urologist
is the primary caregiver who diagnoses
the disease and may have removed the
prostate. These patients will continue to
depend on their urologists when problems
and complications develop from
the prostate cancer, such as urinary tract
obstruction, stinting and transurethral
resections of the prostate.
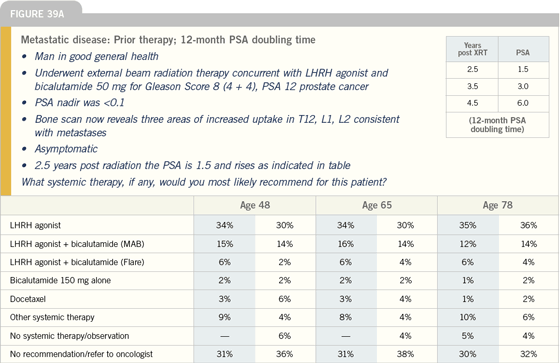
Click here to see image
Incorporating chemotherapy into
the treatment of prostate cancer
| Prostate Cancer Update 2005 (1) |
DR DICKER: Two trials reported at ASCO
2004 demonstrated a survival advantage
in patients with hormone-refractory
disease receiving docetaxel-based
therapy. Docetaxel is being extensively
evaluated in clinical trials in patients
with metastatic disease that is not
hormone refractory. Various randomized
trials are evaluating hormones with
or without chemotherapy in the nonrefractory
population. We don’t know if
chemotherapy — particularly docetaxelbased
chemotherapy — combined with
hormones is beneficial in patients with
locally advanced disease. Chemotherapy
regimens involving taxanes and estramustine
have been evaluated, but estramustine
has a number of side effects,
including deep vein thrombosis. Those
studies have been plagued with toxicities
and haven’t really moved forward.
| Prostate Cancer Update 2005 (1) |
DR PETRYLAK: Our first studies evaluating
docetaxel with estramustine were
performed in the laboratory in 1995. We
were excited by what we saw in vitro and
moved forward into a Phase I study that
opened in February of 1996.
One of the old jokes about Phase I
studies is that the first patient responds
but then nobody else does. Well, the
opposite happened in that study: The
first patient didn’t respond, but nearly
every subsequent patient did. We saw
promising responses in patients who
were heavily pretreated. Median survival
was close to 24 months, and that was the
highest reported median survival of any
study at that time.
This background provided the basis
for SWOG-9916, which is a randomized
trial comparing docetaxel/estramustine
to mitoxantrone/prednisone in men
with progressive androgen-independent
prostate cancer and soft-tissue or bony
metastases. These were not the asymptomatic
patients with rising PSA only.
They had to progress by one of three
criteria: bone scan, CT or PSA. The
trial opened in October 1999 and closed
in January 2003. We demonstrated a 20
percent reduction in the rate of death
in favor of those patients who received
docetaxel/estramustine; however, estramustine-
related toxicity was problematic and included deep venous thromboses,
cardiovascular events and nausea.
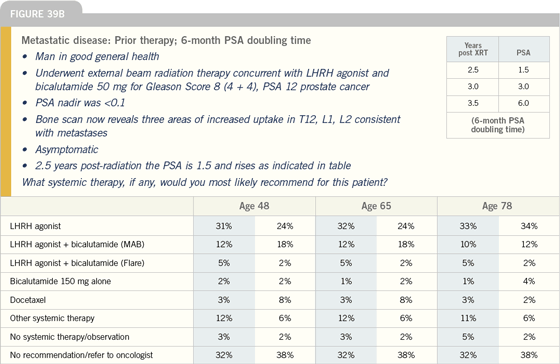
Click here to see image
A related and important trial was
TAX-327, which compared docetaxel
weekly or every three weeks plus prednisone
to mitoxantrone/prednisone. Survival
was improved with every threeweek
docetaxel. The data from both
studies demonstrate for the first time
that we have a chemotherapeutic agent
— docetaxel — that results in prolonged
survival for men with hormone-refractory
prostate cancer.
Because the estramustine-related toxicity
was problematic and the median
survival and hazard ratios are similar
for docetaxel/prednisone and docetaxel/
estramustine, the FDA has recommended
docetaxel/prednisone as the standard
of care for hormone-refractory metastatic
prostate cancer.
The FDA approved docetaxel for
patients with hormone-refractory metastatic
prostate cancer but didn’t specify
when it should be utilized. Hormonerefractory
prostate cancer is a continuum.
In general, the first sign of disease
breakthrough is a rising PSA, and the
patient is often asymptomatic. Generally,
after seven to 12 months, we start seeing
changes in scans, and patients become
symptomatic. A window exists during
which markers are going up and the
patient is asymptomatic, yet the patient
may want treatment.
Often physicians will try a second
hormonal manipulation, such as ketoconazole,
high-dose bicalutamide or
nilutamide. All of these seem to have a
20 percent to 40 percent rate of response
and a median time to progression of
about four months, but no proven survival
benefit.
An interesting observation gleaned
from a subanalysis of TAX-327 data is
that the hazard ratios for survival are
similar whether patients are asymptomatic
or symptomatic, and the difference
of two months in median survival is conserved
for both symptomatic and asymptomatic
patients.
It is difficult to decide whether to
utilize docetaxel in patients who are
asymptomatic but have rising PSAs. It
is important to evaluate how rapidly the
disease is progressing. Clearly, if the PSA
is not rising rapidly, you have time to try
other manipulations. In my experience,
by the time those manipulations fail,
patients need chemotherapy.
In asymptomatic patients with rapidly
rising or rapidly doubling PSA levels,
progression of soft-tissue disease or
progression on bone scan, I consider
initiating chemotherapy. During the initial
PSA rise, unless the patient has
visceral disease, I’m not in favor of using
chemotherapy. I would utilize an investigational
agent or a secondary hormonal
manipulation.
To use a baseball analogy, docetaxel
can be saved as the “relief pitcher” for late
innings, or you can use it earlier as your
starting pitcher. Either way, we know
that docetaxel has a high response rate and a proven survival benefit.
Click here to see image
TAX-327: Docetaxel/prednisone
versus mitoxantrone/prednisone
| Prostate Cancer Update 2005 (3) |
MARIO A EISENBERGER, MD: The patients
enrolled in this trial had hormone-refractory
metastatic prostate cancer and a
testosterone level in the castrate range.
Patients were allowed to have received
only one prior chemotherapy treatment
with estramustine and were withdrawn
from anti-androgen therapy. The trial’s
endpoint was survival. We wanted to
detect a hazard ratio of 0.75 for survival
in favor of docetaxel.
The three treatment arms included:
(1) docetaxel 75 mg/m2 every three
weeks plus prednisone, (2) docetaxel 30
mg/m2 weekly for five out of six weeks
plus prednisone and (3) mitoxantrone
12 mg/m2 every three weeks plus prednisone.
We enrolled 1,006 patients over
two years, and the analysis occurred
about three and a half years after the first
patient was enrolled. Each treatment
arm had more than 300 patients.
With a median follow-up of about
20.7 months, the median survival for
patients treated with every three-week
docetaxel and prednisone was 18.9
months, compared to a median survival
of 16.5 months for those treated with
mitoxantrone and prednisone. Forty-five
percent and 48 percent of patients treated
with every three-week and weekly
docetaxel had a 50 percent decline in
their PSA that lasted for at least three
weeks, and 32 percent of the patients
treated with mitoxantrone and prednisone
had a 50 percent decline in their
PSA, which was significantly different
(p < 0.001).
About 30 percent of the patients in
the docetaxel arms had a reduction in
pain, compared to about 20 percent of
the patients treated with mitoxantrone
and prednisone. The difference in the
reduction in pain between mitoxantrone
plus prednisone and every three-week
docetaxel plus prednisone was also significant
(p = 0.01). Very few objective
responses in soft tissue metastases were
reported in all three arms.
The toxicity was as predicted with
these compounds, mostly myelosuppression.
Thirty-two percent of the patients
treated with every three-week docetaxel
and prednisone had myelosuppression
(Grade III/IV neutropenia), but less
than three percent had neutropenic fever,
documented sepsis or death. Only 1.5
percent of the patients receiving weekly
docetaxel and prednisone had myelosuppression
(Grade III/IV neutropenia),
compared to about 20 percent of the
patients on mitoxantrone and prednisone.
The incidence of febrile complications
was very low and similar in all three
treatment arms.
The other toxicities were minor
(≤Grade II) and not dose limiting. There
was some neuropathy, fatigue and edema
in the patients treated with docetaxel, which is more toxic than mitoxantrone
and prednisone, but the toxicities were
quite reasonable. We had very few episodes
of significant nausea and vomiting
and some changes in liver function
tests. Alopecia was reported more frequently
with every three-week docetaxel,
and changes in the nails and eyes were
reported more with weekly docetaxel.
About 16 percent of the patients on
weekly docetaxel discontinued treatment
because of an adverse drug reaction,
compared to only 11 percent on every
three-week docetaxel.
Click here to see image
Click here to see image
Click here to see image
Comparing the results
from SWOG-S9916 to
those from TAX-327
| Prostate Cancer Update 2005 (3) |
DR CRAWFORD: We reported at a plenary
session at ASCO 2004 and published
in The New England Journal of Medicine
our large Phase III trial headed up by
Dan Petrylak from Columbia, which
compared docetaxel and estramustine to
mitoxantrone and prednisone.
This was the first trial in my history
in the Southwest Oncology Group to
show a survival benefit, and we’ve studied every drug known to mankind. While
the survival benefit in SWOG-S9916
was a couple of months, it’s a big leap.
The next leap, I think, is to use that as a
basis for a platform to add new agents.
Click here to see image
| Prostate Cancer Update 2005 (3) |
DR EISENBERGER: The results from those
two trials are very similar. In SWOG-S9916,
survival for the patients treated
with docetaxel plus estramustine was
17.5 months compared to 15.6 months
for those on mitoxantrone plus prednisone.
A PSA response occurred in 50
percent of the patients on docetaxel plus
estramustine, compared to 27 percent of
those on mitoxantrone plus prednisone.
The difference between the two trials
was in the toxicities. Although no head-to-
head comparison was conducted,
estramustine plus docetaxel was more
toxic than docetaxel plus prednisone.
The most significant toxicities were
cardiovascular or thrombotic. Halfway
through SWOG-S9916, the protocol
was amended to include prophylactic
anticoagulation (ie, warfarin plus aspirin)
for the patients treated with estramustine.
Click here to see image
Click here to see image
Click here to see image
| Prostate Cancer Update 2005 (2) |
DR D’AMICO: In 2004, results from two
trials comparing docetaxel-containing
regimens to mitoxantrone with prednisone
in patients with hormone-refractory
metastatic prostate cancer were
published in The New England Journal
of Medicine — one was SWOG-9916 by Dr Dan Petrylak, and the other was
TAX-327 by Dr Ian Tannock. Both
studies demonstrated a survival benefit
of about two months for the docetaxelcontaining
regimen.
One study combined estramustine
with docetaxel, and the other evaluated
docetaxel alone. Both studies showed
a similar prolongation in survival, but
because estramustine increased toxicity,
it is not considered a necessary part of
the regimen. Two dosing regimens for
docetaxel were evaluated: every three
weeks and weekly. The every three-week
regimen appeared to be better, although
the FDA and others are going to validate
that in the future. The currently accepted
regimen for docetaxel is 75 mg/m2 every three weeks.
Patients whose performance status is
good — such as men under 65 years of
age — will tolerate docetaxel well. They
come in, receive the infusion, go home,
have a couple of days with some symptoms
and then go back to their routine.
Toxic deaths are rare and few patients
require hospitalization for complications.
Growth factors can be used to bring
up blood counts if need be, and these
patients must have their blood counts
monitored. This is a new arena, not for
medical oncologists, but for the urologists
and radiation oncologists who deal
with patients with prostate cancer.
| Prostate Cancer Update 2005 (2) |
DR GOMELLA: The new data showing a
survival advantage with docetaxel-based
chemotherapy in patients with hormonerefractory
prostate cancer are provocative.
The two large trials reported at
ASCO in 2004 have made early chemotherapy
a more viable option. The tolerability
of docetaxel is also significantly
better than the estramustine-based therapies
that caused so much toxicity in the
1990s.
At this time, the average patient with
a PSA recurrence who has not demonstrated
metastatic disease is treated
with hormonal therapy front line and,
if that fails, another hormone intervention
second line. My third-line treatment
is chemotherapy, because I believe our
best opportunity to intercede and have a
favorable outcome is in the earliest stages
of progression.
For example, we learned that salvage
radiation therapy after radical prostatectomy
is more effective when used earlier
rather than later. We used to initiate
salvage therapy when the patient’s PSA
reached 4 ng/mL, then 2 ng/mL, then 1.5 ng/mL. Now, for the best outcome,
we initiate salvage radiation when the
PSA reaches 1 ng/mL. I believe using
chemotherapy earlier in the disease is
reasonable to consider, although we don’t
have any good studies yet to say it should
be utilized at the first evidence of PSA
recurrence.
We are also seeing an emphasis on
a multidisciplinary team approach and
consulting with the medical oncologist
earlier in the management of prostate
cancer. Previously, we didn’t have effective
chemotherapy regimens to offer patients
— nothing demonstrated a statistically
significant advantage in large prospective
randomized trials until mid-2004,
when the two positive docetaxel studies
were reported. I believe we will see an
intrinsic change in the management of
this disease as a result of these data. In
addition, other compounds will be available
in the next couple of years that may
further redefine how patients with PSA
recurrence or progressive prostate cancer
are managed.
| Prostate Cancer Update 2005 (2) |
DR DREICER: Although I was obviously
delighted to see the results of SWOG-S9916
and TAX-327, in effect, they’ve
created many more questions than were
answered. The majority of the patients
enrolled in the two trials had androgen-
independent metastatic disease, and
many, but not all, were symptomatic.
Until those data were available, chemotherapy
in a noninvestigational setting
was used to palliate patients; therefore,
most patients, at least theoretically, were
treated when they had disease-related
symptoms.
The question now is, Does the patient
who has asymptomatic metastatic disease
need to be treated at that time,
or later? That’s a critical question to
which we don’t know the answer. In my
practice, for asymptomatic patients with
low-volume disease, I have a discussion
about what we know about the trials. As
an academician, I have clinical research
opportunities for some of these patients
and certainly would steer them in that
direction. When a patient is not interested
in participating in a clinical trial,
I review the data with him and try to
arrive at a reasonable decision based on
his individual perspective.
| Prostate Cancer Update 2005 (3) |
DR KLOTZ: Among patients with metastatic
disease, two trials have shown
a survival benefit with docetaxel. This
was widely acknowledged to be a huge
step forward because, up to that point,
chemotherapy provided just a quality-oflife
benefit. A survival benefit is a major
event. Of course, the size of that benefit
was somewhere around two and a half
months. The trials compared docetaxel
against other chemotherapy regimens.
Hence, it’s definitely a significant event
in the history of the management of
prostate cancer.
Clearly, the standard of care is now
docetaxel, and it should be offered to
patients who have hormone-refractory
metastatic prostate cancer. The controversy
involves whether it should be
offered earlier, and those studies are
being conducted. If a patient has a rapidly
rising PSA with hormone-refractory
metastatic disease — whether he’s symptomatic
or not — I think it’s reasonable
to treat him with docetaxel. I use the
PSA doubling time as a surrogate marker
for symptomatic progression, because
I know that the patient is going to have
symptoms soon. If he has hormonerefractory
disease without evidence of
recurrence, I don’t treat him.
Docetaxel is very well tolerated,
and the mortality rate from toxicity
is extremely low. I have been very
impressed with the favorable toxicity
profile of docetaxel. I also think that
elderly patients tolerate it quite well. The
toxicity associated with chemotherapy is
acute, while the toxicity associated with
hormonal therapy is chronic, long term
and insidious. Patients receive chemotherapy
for a much shorter period of
time, as a rule.
Select publications
|

