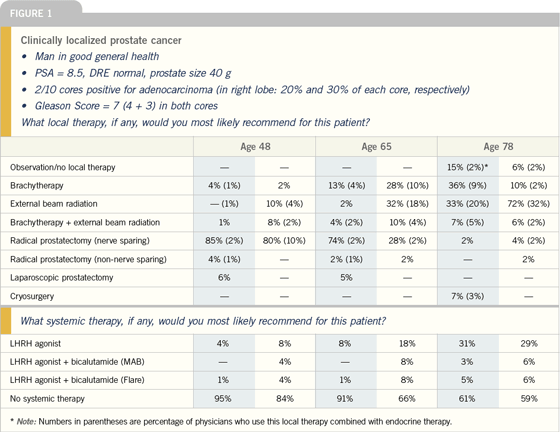
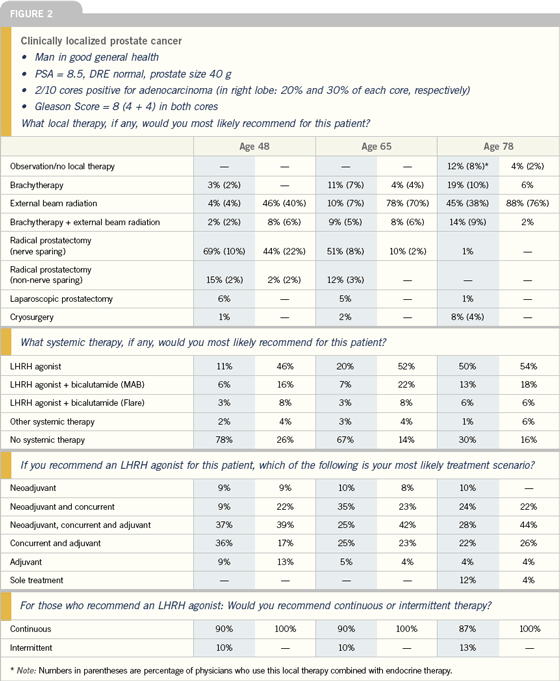
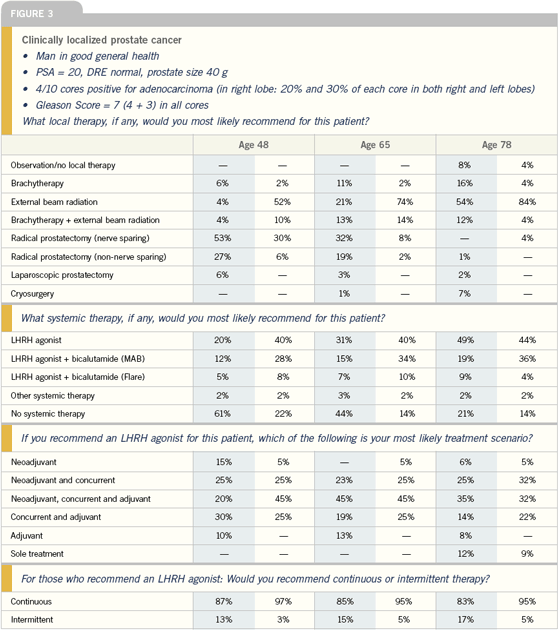
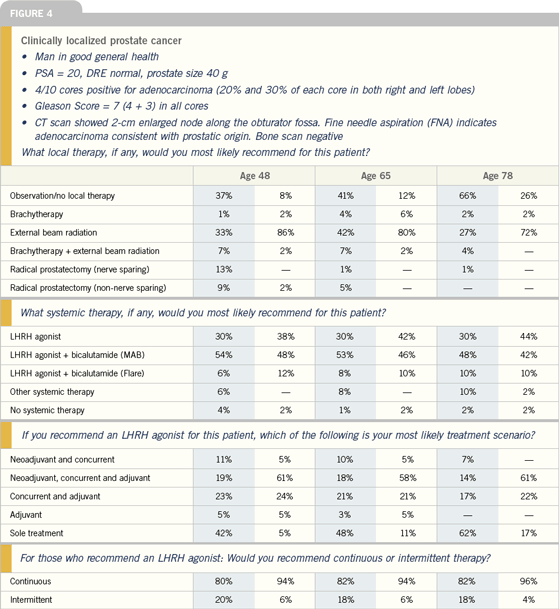
| |
Primary Therapy for Intermediate and High-Risk Disease |

Click here to see image
Hormonal therapy combined
with radiation therapy
| Prostate Cancer Update 2005 (2) |
ANTHONY V D’AMICO, MD, PHD: The
study we published in JAMA was a
randomized trial with 206 men comparing
3D-conformal external beam radiation
therapy (total dose of 70.35 Gray)
with or without six months of combined
hormonal blockade administered for two
months before, two months during and
two months after radiation therapy.
In the study, 57 percent of the patients
had a PSA that was greater than 10
ng/mL, and 73 percent of the patients
had a Gleason Score of seven or higher.
This was a study of patients with highgrade
cancer. For the most part, patients
had T1c disease. More than half the
patients had PSA-detected disease and
about 50 percent had T2 or palpable
tumors.
The primary endpoint of the trial was
progression-free survival. Because the
effect of hormonal therapy on cancerrelated
death was higher than expected,
we saw a difference in overall survival,
just like the Bolla trial. At five years,
progression-free survival was 82 percent
for the patients treated with hormonal
therapy plus radiation therapy versus 57
percent for those treated with radiation
therapy alone. This means the patients
treated with radiation therapy alone had
a PSA elevation and were on hormonal
therapy 25 percent more frequently.
Cancer-specific mortality at five years
was zero in the patients treated with
hormonal therapy plus radiation therapy
versus six percent in the patients treated
with radiation therapy alone; overall
survival demonstrated a 10 percent difference
(88 percent versus 78 percent,
respectively). The absolute number of
deaths due to prostate cancer was six
in the radiation therapy-only arm and
zero in the hormonal therapy plus radiation
therapy arm. Out of 206 patients,
a six-event difference in prostate cancer
deaths was enough to account for a survival
difference, mainly because we initially
screened patients for cardiovascular
disease. The hazard ratio for overall survival was two, which means a twofold
reduction in deaths in the men randomly
assigned to combined hormonal therapy
plus radiation therapy.
Click here to see image
Click here to see image
The validation that the combination
of hormonal therapy and external beam
radiation therapy provides a survival benefit
compared to radiation therapy alone
is an important clinical message.
A number of randomized studies have
evaluated this comparison, particularly
in men with localized high-risk disease.
Click here to see image
“High risk” in this scenario is defined as
a Gleason Score of seven or higher or a
PSA level greater than 10 ng/mL.
The most recent study, published in JAMA in August 2004, demonstrated
a 10 percent survival benefit at five
years for men who received six months
of hormonal therapy in combination
with radiation therapy compared to men
who received radiation therapy alone.
Hormonal therapy consisted of flutamide
with either leuprolide or goserelin.
Two questions remain in this scenario:
(1) Is combined hormonal blockade
necessary? and (2) Are six months of
hormonal therapy adequate in patients
with Gleason eight, nine or 10 disease,
even if it is T1c or T2?
Click here to see image
The studies preceding the trial published
in JAMA were RTOG-9202 and
the Bolla trial. The Bolla trial — an
EORTC study — found that three years
of hormonal therapy is better than no
hormonal therapy. RTOG-9202 found
that two years and four months was better
than just four months of hormonal
therapy. It was not an overall survival
benefit but a cancer-specific survival benefit
of 3.4 percent at five years.
The question still remains whether
long-term hormonal therapy is necessary
and safe. A European randomized study
comparing three years to six months of
hormonal therapy should answer the
question more definitively. If long-term
hormonal therapy truly is better, I suspect that older men (over 70 years of
age), in whom occult cardiovascular disease
can be prevalent, will benefit least,
whereas younger men who don’t have
cardiovascular issues may benefit most.
Click here to see image
| Prostate Cancer Update 2005 (3) |
ANTHONY L ZIETMAN, MD: RTOG-8610
and RTOG-9202 are maturing. Every
time they’re republished, the benefit
from adjuvant androgen deprivation
therapy seems to be confirmed. We now
think about the use of adjuvant androgen
deprivation with radiation therapy
as follows: Patients with low-risk disease
don’t need it, and patients with high-risk
disease do. We can probably use less
hormonal therapy if the patient has a
Gleason seven tumor and a PSA below
20 ng/mL. The patient needs more,
maybe two or three years of hormonal
therapy, if he has both a high Gleason
Score and PSA.
The patients with intermediate-risk
disease are an intriguing group. Anthony
D’Amico published in JAMA 2004 the
results from a randomized trial in which
a little over 200 men with intermediate-
risk prostate cancer were randomly
assigned to receive radiation therapy
alone or with six months of hormonal
therapy. Combined androgen blockade
was administered two months before,
two months during and two months
after conventional-dose radiation therapy.
Not only did the trial show a diseasefree
survival advantage, but it has also
shown an overall survival advantage at
only five years.
| Prostate Cancer Update 2005 (1) |
ADAM P DICKER, MD, PHD: In radiation
oncology, it’s almost a mantra that if
we don’t achieve local control, we won’t
achieve distant control. This is not only
true in prostate cancer; it’s also true
in breast cancer. Zietman published an
article stating that the metastasis rate in
prostate cancer is increased when local
control is not achieved. Twenty years
ago, everyone treated the whole pelvis
with radiation to the nodes because it
was believed that is where prostate cancer
spreads; however, that practice was not
based on any evidence. Roach’s Phase
III trial, RTOG-9413, comparing whole
pelvic to prostate-only radiation therapy
and neoadjuvant to adjuvant combined
androgen suppression, was the first to
demonstrate that large-field radiation
therapy with neoadjuvant and concurrent
hormonal therapy had a benefit as
measured by PSA.
It appears radiation therapy will
probably cure microscopic disease in the
nodes, but only when combined with
hormonal therapy. I don’t anticipate that
radiation therapy alone — at the dose we
used, which was limited because of the
small bowel — will cure micrometastatic
disease. Some people believe hormonal
therapy is synergistic with radiation. I’ve
seen no evidence of that; rather, it probably
has an additive effect.
I would not use the term “radiosensitizer”
because hormonal therapy is active
by itself, but it certainly augments radiation.
I believe hormonal therapy plays
a role, but how much of a role it plays
locally is unclear. It’s also not clear that
the dose used in the Bolla study is sufficient
to cure patients. A number of
investigators are retrospectively examining
their data from patients who received
a Bolla-like therapy in various doses during
different time periods to determine
whether an increase in dose translates to
decreased bony metastases and improved
survival.
| Prostate Cancer Update 2005 (4) |
MACK ROACH III, MD: The CaPSURE
database would suggest there’s been a big increase in the use of hormone therapy
with radiation therapy — appropriate
use, by and large, in the sense that
patients with intermediate and high-risk
disease are receiving it. Some individuals
are probably still being treated inappropriately
because they are receiving
hormone therapy for low-risk disease.
Some of those may be patients who were
trying to decide what they wanted to do
and were feeling nervous and wanted
to try something because they didn’t
want to remain untreated. Some of the
patients with low-risk disease may have
received hormone therapy to shrink the
prostate in preparation for brachytherapy.
However, I do suspect some physicians
still utilize hormone therapy because
they believe that hormone therapy and
radiation therapy go hand in hand. Well,
it turns out that in patients with low-risk
disease, there’s no benefit, and hormone
therapy causes hot flashes, osteoporosis,
fatigue, anemia and impotence. So if you
were not going to benefit the patient in
terms of biochemical control or survival,
I wouldn’t recommend it for patients
with low-risk disease.
Click here to see image
Duration and benefit of
adjuvant hormone therapy
| Prostate Cancer Update 2005 (2) |
LEONARD G GOMELLA, MD: We generally
recommend two to three years of adjuvant
hormonal therapy when treating
patients with locally advanced disease,
based primarily on the data from recent
trials. Bolla’s EORTC trial showed superior
outcomes in patients who received
three years of hormonal therapy, and the
RTOG-9202 showed that two years of
hormonal therapy resulted in a survival
advantage.
Admittedly, the duration of hormone
therapy is controversial. Some institutional
studies have suggested that as
little as six months of hormonal therapy
may be beneficial, and that’s possible, but
our recommendations rely on the larger
multi-institutional trials with thousands
of patients. While the prospective randomized
trials all show this approach
is effective, particularly in patients with
high-risk disease, it’s not advantageous
for all patients. Patients with low-risk
disease do not appear to benefit from the
combination of hormones and radiation,
and the side effects may detract from
the patient’s quality of life and overall
outcome. In the RTOG-9202 trial,
an overall survival advantage was seen
in patients with high Gleason Scores;
however, in the patients with lower-risk
disease, although the combination may
enhance PSA control, we don’t see much
improvement in survival.
| Prostate Cancer Update 2005 (1) |
DR DICKER: A couple of interesting recent
research developments relate to the
management of locally advanced prostate
cancer. First, D’Amico et al reported
results of a clinical trial that randomly
assigned patients to receive radiation
therapy with or without six months of
total androgen suppression.
The group that received hormonal
therapy demonstrated a survival advantage,
which was surprising because the
trial did not accrue a large number of
patients. These data raise the question of
whether six months of hormone therapy
is adequate, or whether we need longer-duration
therapy.
Another related article that touches
on the duration of hormonal therapy is
by a group in Finland who evaluated cognitive
function in men — with an average
age of 65 — before and after six and
twelve months of hormonal therapy.
Click here to see image
They found a significant decline in
memory, time to process information,
recall and visuomotor function associated
with the decrease in testosterone.
Their data do not directly connect
hormonal therapy with the decline in
psychomotor function, but it is clear
to those who treat prostate cancer that
long-duration therapy — more than one
year — impacts patients’ mental acuity.
Clinicians are interested in determining
the maximally effective therapy
that can be delivered with minimal side
effects. When combined with radiation
therapy, total androgen suppression may
be equivalent to longer-duration therapy
with an LHRH agonist alone for the
treatment of clinically localized prostate
cancer.
| Prostate Cancer Update 2005 (4) |
DR ROACH: The patients with high-risk
disease who are treated with external
beam radiotherapy have been shown to
benefit from long-term hormone therapy.
The question of how long they should
be treated is controversial.
Although it’s commonly done, no randomized
trials have used one year of
adjuvant hormone therapy. The shortest
duration shown to improve survival was
two years, and that was in RTOG-9202.
The EORTC study reported by Bolla
used three years of hormone therapy,
and RTOG-8531 used hormone therapy
for life.
In my opinion, recommending one
year of adjuvant hormone therapy is
experimental because it has not been
proven. I think it may turn out that one
year is as good as three years or four
years, but in breast cancer, a longer duration
of hormone therapy has been shown
to be better than a shorter duration of
hormone therapy. So in prostate cancer,
I think patients with high-risk disease
should be offered two years or more of
adjuvant hormone therapy.
The most common duration of hormone
therapy that I recommend for the
typical patient who has T1/T2 disease
and a PSA around 10 ng/mL is two
years, although there are some patients
with whom I do not feel comfortable
stopping after two years. I have one
patient whose PSA was in the thousands,
and he had lymph node involvement.
At present, he’s doing fine. He’s
out three years, and his PSA is undetectable.
However, I am afraid to discontinue
his hormone therapy.
I suspect it’s possible he’ll be on hormone
therapy for the rest of his life.
But in the typical patient who’s been
screened, detected early and treated
aggressively with local-regional therapy,
including lymph node radiation, my
standard use of adjuvant hormone therapy
is for two years.
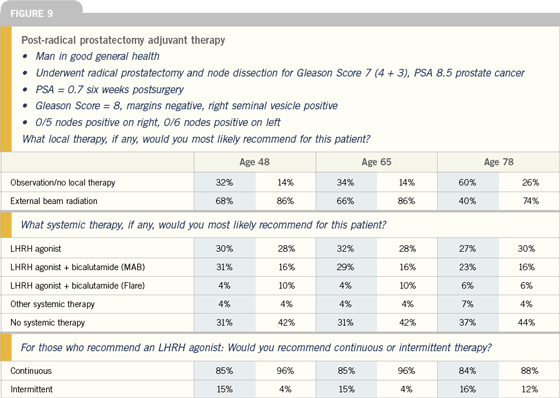
Click here to see image
Intermittent hormonal therapy
| Prostate Cancer Update 2005 (2) |
DR GOMELLA: Intermittent hormonal
therapy is not considered the standard of
care, but we do use it in select patients.
The data on this therapy are conflicting
— some preliminary European studies
show that it doesn’t adversely affect overall
PSA recurrence or survival, whereas
other studies report adverse outcomes in
prostate cancer progression with intermittent
therapy.
One of the challenges is that we are
waiting for data on intermittent therapy
from the large ECOG trial completed
in the United States several years ago.
The problem is that this trial evaluated
intermittent therapy in patients with
high PSA levels and metastatic disease.
Most of us believe that intermittent
therapy will probably be most effective
in patients with a low disease burden and
minimal PSA elevation.
In fact, we know from the Messing
trial that some patients with micrometastatic
disease receive hormonal therapy
and never have a recurrence.
Certainly some patients who choose
to discontinue hormonal therapy will
not have disease relapse. This is anecdotal,
but I have two young patients
who had node-positive, micrometastatic
disease with undetectable PSAs postoperatively.
After approximately three years of
adjuvant hormonal therapy, they each
asked me to take them off of hormonal
therapy. They are now approaching
almost 10 years since their diagnosis
with no evidence of recurrence and they
both have normal PSA levels.
What we really need are more studies
on intermittent therapy for PSA-only
recurrences with low levels. Because we
don’t have the data, we can’t recommend
intermittent therapy as a definitive treatment
option; however, we can certainly
discuss it with patients.
Neoadjuvant hormonal
therapy trials
| Prostate Cancer Update 2005 (3) |
E DAVID CRAWFORD, MD: Years ago, studies
of hormonal therapy administered
prior to radical prostatectomy were
conducted to determine if the positive
surgical margin rate could be improved.
Dr Soloway did a study in the United
States, as did Dr Debruyne in Europe
and Dr Gleave in Canada.
These studies showed that the use
of hormonal therapy for three or eight
months before surgery significantly
decreased the positive margin rate.
However, at three, five and even six
years, no differences in the PSA failure
rates were noted, which led people to believe that neoadjuvant hormonal
therapy didn’t do anything. These trials,
however, were not powered with enough
numbers and follow-up time.
My prediction is that with time, some
of these studies will show a difference in
the PSA failure rates. In patients with
local prostate cancer, we’re not going to
obtain answers quickly. Sometimes it
will take 10 or 15 years to observe a difference
between the arms of a trial.
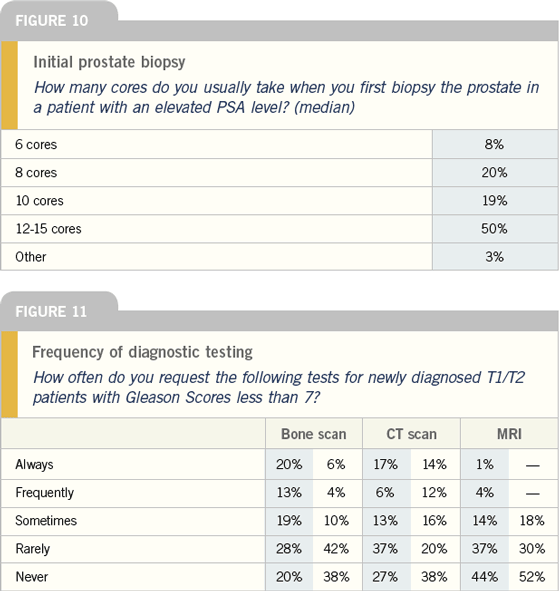
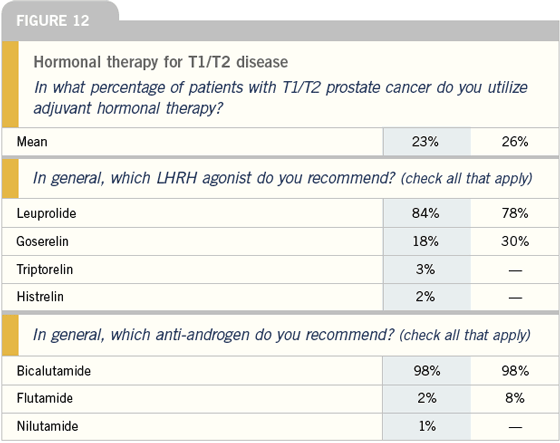
Adjuvant hormonal therapy in
breast and prostate cancer
| Prostate Cancer Update 2005 (1) |
PROFESSOR SIR RICHARD PETO: Various
reasons exist for the difference in the
clinical research data between prostate
cancer and breast cancer. First, breast
cancer occurs in younger women while
prostate cancer occurs in older men.
Obviously, a patient with a 40-year
life expectancy is more interested in what
happens in the long term than a patient
with a 10-year life expectancy.
Second, the early hormonal treatments
for prostate cancer were unpleasant.
They consisted of castration and
diethylstilbestrol (DES), which was discovered
to be seriously cardiotoxic and
would actually do more harm than good
in terms of life expectancy. As soon as
DES was no longer used and alternative
means of turning off testicular function
were discovered, trials began.
Hormonal therapy for prostate cancer
substantially delays progression of
the disease and moderately delays death
from the disease. The effects of immediate
hormonal treatment versus deferred
hormonal treatment in a man with prostate
cancer are comparable to the effects
of five years of adjuvant tamoxifen in a
woman with hormone-sensitive breast
cancer. Additionally, hormonal therapy
prevents a number of complications of
metastatic disease, such as spinal metastases,
ureteric obstruction and the need
for further surgery.
The prostate cancer trials were not
as large as the breast cancer trials, so the
results were muddled by deaths from other causes. The curves are similar,
but the prostate trials have statistical
noise from the large numbers of deaths
that are unrelated to prostate cancer or
its treatment. When patients are older,
deaths from other causes confuse trial
results.
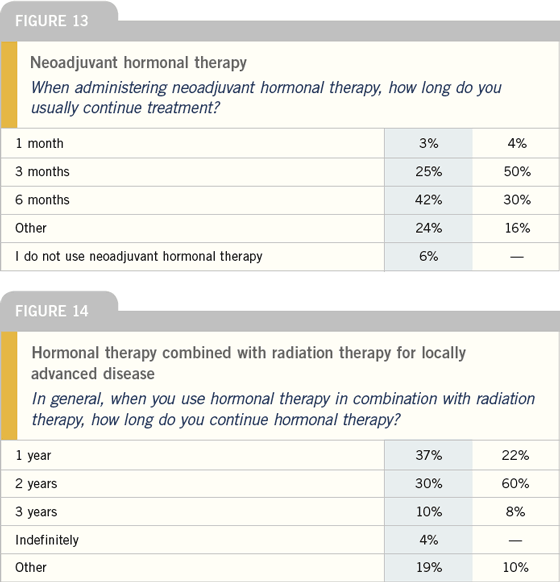
The problem with evaluating hormone
therapy for prostate cancer is that
only a few thousand men with prostate
cancer were being randomly assigned to
therapy, compared to tens of thousands
of women with breast cancer. That is
why the evidence of benefit in breast cancer
is so much better.
In breast cancer, we have seen impressive
decreases in death rates in middleaged
women as a result of early use of
tamoxifen and chemotherapy. I believe
the effects of earlier treatment with hormonal
therapy in prostate cancer over
the next five or 10 years will be comparable
to those produced by tamoxifen in
breast cancer.
No good evidence indicates that
bicalutamide treatment affects mortality
from causes other than prostate cancer.
Currently, the number of deaths from
prostate cancer in the EPC trials is so
limited that it is difficult to obtain any
clear evidence of an effect on prostate
cancer mortality. The question as to the
effect on overall mortality is well worth
asking, but it needs to be answered by
separate analyses of prostate cancer mortality
and nonprostate cancer mortality.
One should ask, “Is there any evidence
of hazard?” No. “Is there any evidence
of benefit?” At some point, the answer
to that question may well turn out to
be “yes.”
No good evidence indicates that
bicalutamide increases the overall death
rate from causes other than prostate
cancer. If you have no overall evidence
and you begin looking for subgroups of
this and subgroups of that, you’re almost
bound to find a subgroup in which the
results seem favorable and a subgroup in
which the results seem unfavorable, but
that is just statistical noise.
Role of combined
hormonal blockade
| Prostate Cancer Update 2005 (2) |
DR D’AMICO: Off protocol, in patients
with high-risk T1c or T2 disease, I use
six months of combined hormonal blockade
because that is what I used in the
study I conducted.
In that study, about 27 percent of the
patients did not complete the six months
of flutamide, mainly because of elevations
in their liver function tests (LFTs).
They weren’t necessarily having toxicities
from the flutamide, but we had a
rule: If the LFTs exceeded two times the
upper limit of normal, we discontinued
the drug for that patient. Despite that,
the survival benefit was still seen.
It is an open question whether combined
hormonal blockade is really necessary;
however, without an answer from a
randomized trial, I follow the randomized
trial results we have.
When we designed that trial in 1994,
bicalutamide wasn’t available, so flutamide
was used. Today, bicalutamide is
used because it’s a once-a-day drug and it
doesn’t have the same LFT issues.
In patients who have T3 or T4 disease
by palpation, I use exactly what
the RTOG utilized in their randomized
study: two months of neoadjuvant combined
hormonal blockade, two months
of combined hormonal blockade concurrent
with radiation therapy and two
years of an LHRH agonist alone.
| Prostate Cancer Update 2005 (4) |
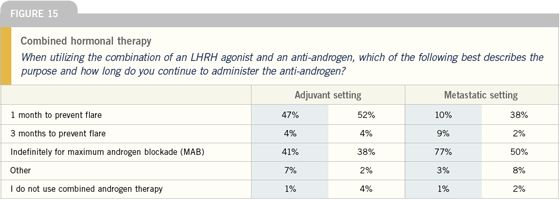
DR ROACH: We generally utilize monotherapy,
because the trials have evaluated
monotherapy in the adjuvant setting.
However, the trial that studied two
years of adjuvant hormone therapy used
combined blockade for four months.
The EORTC study, which looked at
three years of hormone therapy, utilized
combined blockade for one month. In
RTOG-8531, in which they study adjuvant
hormone therapy for life, they do not utilize combined hormone blockade.
Most patients would prefer to take
combined androgen blockade for four
months and two years of hormone therapy.
In 2000, we published a paper — the
“meta-analysis” of the RTOG trials. We
took all of the trials, pooled them together,
and stratified them by their risk of
death. We concluded that patients with
low-risk disease did not benefit from
hormone therapy. Patients with intermediate-
risk disease — very much like
Dr D’Amico’s patients with intermediate-
risk disease, except maybe a little
bit higher risk — benefited from shortterm
hormone therapy (four months,
for example), and patients with highrisk
disease needed long-term hormone
therapy.
Click here to see image
SWOG-S9921: MAB with
or without mitoxantrone/
prednisone after prostatectomy
Prostate Cancer Update 2005 (3) |
DR CRAWFORD: This ongoing trial, which
is very important, randomly assigns men
who have had a radical prostatectomy
and are at high risk for recurrence to
combined androgen blockade for two
years with or without chemotherapy.
We have almost 500 patients enrolled
on this study, and we need about 1,400
to complete it. SWOG-S9921 sets out
to define whether adding something to
radical prostatectomy makes a difference.
| Prostate Cancer Update 2005 (4) |
IAN M THOMPSON, MD: Clearly, one of the
two most important issues in early prostate
cancer is how best to treat high-risk
prostate cancer. This issue of adjuvant
therapy sits kind of at bookends to the
other important issue, which is how to
determine clinically important prostate
cancer. The issue of adjuvant treatment
for high-risk cancer is the bookend that
highlights the fact that some men clearly
have bad prostate cancer for which
monotherapy is inadequate. The question
is, what should additional therapy
include, be it radiation therapy, hormonal
therapy, chemotherapy, biologic response
modifiers, or combinations thereof?
If you look at the men in the SWOGS9921
study, men tolerate treatment
with mitoxantrone/prednisone extremely
well. The task is explaining to the men
that he needs additional therapy because
he has high-risk disease and that he can
expect to have some hot flashes while on
two years of hormonal therapy. As urologists,
we oftentimes think a cytotoxic is
much worse than an LHRH. However,
in our experience, once men understand
the side-effect profile of the hormonal
therapy and chemotherapy, the two therapies
are seen as approximately equal in
terms of side effects and toxicities.
Ongoing clinical trials evaluating
docetaxel in patients with
earlier-stage disease
| Prostate Cancer Update 2005 (2) |
DR D’AMICO: We are conducting a trial
in patients with high-risk disease.
Patients are treated with radiation therapy
and hormonal therapy with or without
docetaxel. The chemotherapy will
be administered for two cycles prior to
the start of radiation therapy, concurrent
with hormonal therapy and weekly
during radiation therapy, so it’s approximately
four months of chemotherapy.
Dr Howard Scher is conducting a
trial of hormonal therapy with or without
docetaxel in patients with rapidly
rising PSAs (eg, doubling times less than
three to six months) following surgery or
radiation therapy. Dr Mario Eisenberger
will be conducting a postoperative adjuvant
study in men with high-risk features
at prostatectomy (ie, seminal vesicle invasion,
Gleason Score of 8 to 10); patients
will receive hormonal therapy and be
randomly assigned to docetaxel or no
further therapy.
It’s important to select patients carefully
for these studies. For example, the
vast majority of patients with a PSA
failure after local therapy don’t die from
prostate cancer. We know now that it’s the rate of rise of the PSA — and not
the PSA failure itself — that’s important,
so patients whose PSAs are rising
quickly are the patients you want to
enroll in these studies. The toxicity from
chemotherapy occurs up front, and even
younger men require some down time
during the chemotherapy regimen. They
have to be willing to accept an acute
decrement in quality of life for a benefit
that’s not yet proven.
The study I’m conducting in men
with high-risk disease is powered for a
hazard ratio of 1.5, whereas the hazard
ratio in our study with hormonal therapy
was two. With the chemotherapy, we’re
hoping to see half the improvement that
we saw with hormonal therapy. If we had
a 10 percent benefit from hormonal therapy
at five years, we’d be happy with a
five percent benefit from chemotherapy.
I’m powering the study for survival,
pending the validation of a surrogate (eg,
progression-free survival). We evaluated
progression-free survival in the study of
hormonal therapy and radiation therapy
because, when that trial was designed
in 1994, that endpoint was in vogue
for hormonal therapy. The benefit from
hormonal therapy was more than expected.
We also saw a difference in survival;
however, no data for chemotherapy in
localized prostate cancer in a randomized
setting indicate that progression-free
survival can be used as an endpoint.
In our trial, prevention of bone metastases
is a secondary endpoint that is clinically
relevant. If you design a prostate
cancer clinical trial powered for survival,
you’ll have plenty of power to go back
and evaluate progression-free, diseasefree
and cancer-specific survival. But if
you power the trial for an earlier endpoint,
you may not have enough power
to evaluate the ultimate endpoint. We
expect this study will accrue in two years
and be reported three to five years later.
Issues in radiation therapy
| Prostate Cancer Update 2005 (3) |
DR ZIETMAN: Every five years, the Patterns
of Care Study (PCS) group surveys
about 60 academic or community-based institutions across the United States.
It reviews five to 10 randomly chosen
patients from each institution to obtain
a snapshot of what’s going on nationally.
In 2004, it reported the 1999 data
and compared them to the 1994 data.
Hormonal therapy is being used more
frequently with radiation therapy in patients with localized prostate cancer,
indicating the penetration of randomized
trial data into clinical practice. When we
break out hormonal therapy use by low-,
intermediate- and high-risk prostate
cancer, we find many men with low-risk
disease who are receiving hormonal therapy
with radiation therapy, a situation
for which we have no randomized trial
data showing any clear advantage.
High doses of radiation are now more
frequently used. This trend is actually
ahead of the randomized trial data. Both
the PCS and the CaPSURE database
are showing that external beam radiation
therapy is being used less frequently and
brachytherapy is being used more frequently
in early-stage disease. In 1994, of
the cases treated with radiation therapy
in the United States, only three percent
utilized brachytherapy.
By 1999, it was up to 36 percent, and I
can assure you by now it’s well above that.
The CaPSURE database demonstrates
that external beam radiation therapy is
being used only a third as frequently in
the sites they surveyed.
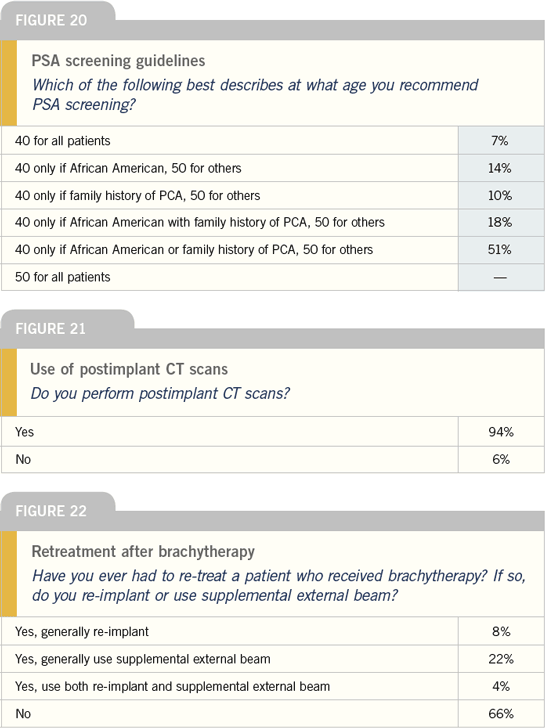

| Prostate Cancer Update 2005 (1) |
DR DICKER: Based on retrospective data
from Richard Stock, we know that to
achieve good biochemical control, 90
percent of the prostate should receive
approximately 145 Gray with an I-125
prostate implant. That doesn’t mean the
patient won’t be cured if only 85 percent
of the prostate is treated, but if a postimplant
CT dosimetry showed only 70
percent or less of the prostate was treated,
I would have some concerns.
It doesn’t matter whether the CT is
performed on the day of the implant or
one month later, but it’s better to receive
feedback as soon as possible after the
implantation. It’s difficult to remember
problems you encountered in the operating
room, especially if you performed
multiple implants on the same day, and
it’s important to understand why one
patient didn’t receive a good dose.
When the prostate implant results in
suboptimal coverage, I tell the patient
we’re not happy with what we achieved
in the operating room and, assuming I understand why things didn’t go well and
the situation can be corrected, my preference
is to reimplant.
Others prefer supplemental external
beam radiation therapy, but it is difficult
to know what dose of radiation
therapy to use. I’ve performed 500 to
600 implants in my career, and I’ve only
had to reimplant twice. Assuming you
didn’t overdose the urethra or the rectum
on the first implant, reimplantation
shouldn’t cause an increase in complications.
| Prostate Cancer Update 2005 (3) |
DR ZIETMAN: Two randomized trials
have compared high-dose to conventional-dose external beam radiation therapy.
The MD Anderson trial evaluated
approximately 300 patients. For the
patients with a PSA > 10 ng/mL, there
was a clear advantage in terms of freedom
from biochemical or disease failure
at five years with high-dose radiation (78
Gray) compared to conventional-dose
radiation (70 Gray). No advantage was
seen for high-dose radiation in patients
with a PSA ≤ 10 ng/mL.
The second randomized trial — the
Massachusetts General Hospital/Loma
Linda University trial — compared highdose
(79 Gray) to conventional-dose (70
Gray) radiation in men who mainly had
low-risk disease. Of the 393 patients
randomly assigned, approximately 250
had low-risk disease. The number of
biochemical failure events at five years
was halved for the patients with low-risk
and intermediate-risk disease who
were treated with high-dose radiation
therapy.
| Prostate Cancer Update 2005 (4) |
DR ROACH: We have looked at our experience
at UCSF in patients treated with
implants versus those treated with external
beam radiation therapy. If you use
endorectal MR spectroscopy and look
at what happens after external beam
radiation therapy versus brachytherapy,
there’s a tendency for the abnormal
spectra to normalize more quickly with
brachytherapy.
If you look at time to complete metabolic
atrophy — the prostate goes from
being active metabolically, which is normal,
to an atrophic state, it is significantly
shorter with a permanent implant. In a
study that observed patients treated with
external beam radiation therapy versus
brachytherapy, we saw about 70 percent
of our patients treated with brachytherapy
had complete metabolic atrophy,
compared to approximately 20 percent
of patients treated with external beam
radiation therapy.
If you evaluate PSA decline and pick
an arbitrary cutoff of PSA less than
0.5 ng/mL, 90 percent of our patients
who were treated with brachytherapy
had a PSA at follow-up of less than 0.5
ng/mL. Less than half of the patients
who were treated with external beam
radiation therapy never got to 0.5 ng/
mL; the median PSA was around 0.9
ng/mL.
The metabolic response of the prostate
is less dramatic with external beam
radiation therapy. The PSA goes down
to a lower level with an implant. This
carries a number of implications. It
doesn’t prove that brachytherapy is more
effective at curing cancer, but it does suggest
that it’s more effective at ablating the
prostate and lowering PSA.
Select publications
|

