| |
Management of PSA-Only Relapse |

Click here to see image
Use of PSA as an endpoint
in clinical trials
| Prostate Cancer Update 2005 (2) |
ROBERT DREICER, MD: With each passing
year, the number of patients with locally
advanced prostate cancer — who are
perhaps destined to do poorly relatively
early — continues to decline as we detect
disease earlier. This impacts our ability
to perform adjuvant studies of chemotherapy.
Currently, the FDA would not
accept PSA failure as a clinical endpoint,
so we have to wait for clinical progression
or death. The FDA is actively
considering these issues, and at least one
forum was held last fall at the FDA, and
another one is planned. Changes may be
occurring in the agency’s attitude toward
PSA as an endpoint, but as of today, it’s
a dilemma. If we can only perform one
study a decade, it will be a long time
before we can answer the question about
adjuvant chemotherapy in the treatment
of prostate cancer.
As a clinical trial endpoint, PSA
remains problematic in some settings. In
patients with biochemical failure only,
using PSA failure as a parameter of
response remains unproven; however, in
the adjuvant setting, I think most of us
who take care of these patients would
clearly accept time to PSA failure as an
endpoint in patients undergoing radical
prostatectomy — albeit not the only
endpoint. Of course, reasonable assurances
must be made to ensure that the
PSA failures are real and not simply low
levels of detectable PSA in patients who
are not destined to progress. That’s the
optimal use of PSA in how we manage
patients today, and it would be problematic
to not use PSA failure as at least an
intermediate endpoint.
Clearly, in studies of hormonal therapy,
PSA failure would not be a useful
endpoint. Biologic or targeted therapies
are also potentially problematic unless we
understand what these drugs do to PSA
expression at the cellular level. With chemotherapy,
we increasingly have reason
to believe it would have validity in the
postprostatectomy setting.
Time to delay of PSA failure is probably
a good surrogate to activity. That’s
not to say you should end the trial based
on that endpoint and not collect other
data, but I believe it’s an endpoint that
will have some value and allow us to
begin testing agents in the adjuvant setting
without having to expose patients
to Phase III investigations. It would
allow us to perform hypothesis-generation studies and select agents that make
rational sense based on some of these
early endpoints and then move on to formal
Phase III studies.
Regarding the metastatic setting,
Dr Crawford presented data at ASCO
2004 that were based on the preliminary
analysis of the SWOG randomized
trial S9916. These data suggested that a
three-month change in PSA was, in fact,
a surrogate for survival in the androgenindependent
setting. Is it the same in
the hormone-naïve environment? I don’t
know, and that’s an important question.
Click here to see image
Use of multiple clinical variables
to predict disease recurrence
J Clin Oncol 2005
We developed a nomogram that estimates
the probability of a positive bone
scan at any time after biochemical failure
before the administration of hormonal
therapy based on commonly available
data, including the results of pathologic
analysis of the operative specimen (status
of surgical margin, presence of extracapsular
extension, seminal vesicle invasion,
and Gleason sum at time of radical
prostatectomy) as well as postoperative
follow-up (tPSA [trigger PSA], PSA
slope, and PSA velocity). The advantage
of this approach is seen in the predictive
ability of our model: bone scan results
were predicted with a concordance index
of 0.93.
— Zohar A Dotan, MD, PhD et al.
J Clin Oncol 2005;23(9):1962-8.
JAMA 2005
A short PSA doubling time (PSADT)
is associated with increased risk of clinical
progression, metastasis, and prostate
cancer–specific mortality. However,
whether other clinical variables add
information to PSADT is less clear.
Using a cohort of patients all having
biochemical recurrence after radical
prostatectomy with prolonged follow-up,
we identified 3 significant risk factors
for prostate cancer–specific mortality:
PSADT, pathological Gleason score, and
time from surgery to biochemical recurrence.
Using these variables, tables were
constructed to estimate the 5-, 10-, and
15-year risk of prostate cancer–specific
survival. …
…The 5-, 10-, and 15-year risk of
prostate cancer survival for a patient not
treated with early hormonal therapy with
a PSADT in less than 3 months, recurrence
3 or more years after surgery, and
a Gleason score between 8 and 10 was 50%, 1%, and less than 1%, respectively.
For a similar patient but with a PSADT
between 3.0 and 8.9 months, the 5-, 10-,
and 15-year risk of prostate cancer survival
was 78%, 19%, and < 1%, respectively.
…Using the clinical variables of
PSADT, Gleason score, and time to
biochemical recurrence, patients could
be stratified into groups with a varying
risk of survival at year 15 of 94% vs
< 1%, although the CIs for many of the
subgroups were large. …
— Stephen J Freedland, MD et al.
JAMA
2005;294(4):433-9.
Click here to see image
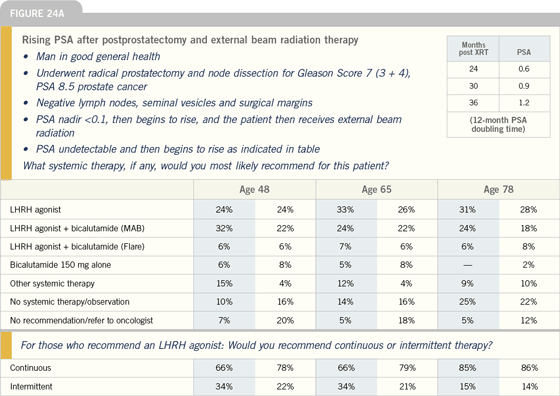
Management of PSA relapse
Prostate Cancer Update
Special Edition 2005
GREGORY S MERRICK, MD: I’m relatively
conservative in managing PSA recurrences.
If we’re going to treat those
patients with hormonal therapy, I do
not recommend the institution of androgen
deprivation therapy until the PSA
doubling time becomes less than 12
months. Once the doubling time is less
than 12 months, I think we have to seriously
consider it.
The big question then becomes continuous
versus intermittent therapy. I
have always been a proponent of intermittent
because it allows a better quality
of life. We like to leave a patient on
therapy for nine to 12 months and, if
the PSA becomes undetectable, to stop
the androgen deprivation therapy until
we once again see the PSA exceed some
arbitrary point, whether it’s 10 or 15
ng/mL.
I believe intermittent androgen deprivation
is a marvelous way to approach
patients with biochemical failures, especially
those who are older, with concomitant
medical problems. We’ve had great
success with watching men along these
lines. They all appear to respond to the
subsequent second or third challenge of
hormonal therapy. The one thing that
you do note, however, is that with each
cycle, the time off hormonal therapy
tends to decrease.
Prostate Cancer Update
Special Edition 2005
DR D’AMICO: In terms of PSA recurrence,
one point that’s become apparent across
the specialties and now is coming into
the community is that the rate of PSA
rise dictates the time interval to a positive
bone scan. For patients with PSA
levels moving rather quickly, even men
in their mid to late seventies, unless they have really significant comorbid illnesses
that are going to take their life this year, I
think it is important to carefully consider
the hormonal therapy.
Another issue is this: When you use
hormonal therapy for a man in the rising
PSA setting, how long should you
administer it? Forever? Intermittently?
For a short course? This question is completely
unanswered. A Portuguese study
was presented at the AUA this year of
intermittent versus continuous therapy
for men with rising PSA or node-positive
or metastatic disease. While the
study only included 800 men, no difference
was seen in overall survival between
intermittent versus continuous treatment.
I will say that with 800 patients, the
trial is likely not large enough to rule out
a small benefit to continuous therapy.
But this is the first small study of intermittent
versus continuous therapy suggesting
equality. Equality in this study
however is probably limited to a five to
seven percent difference. These trials
have to be powered as equivalence studies,
which means they need thousands of
men. The SWOG study is such a study,
but is not yet ready to report. So I don’t
think we’re ready to say intermittent and
continuous therapy are equivalent yet.
But it is a big issue, because lifelong hormonal
therapy in the rising PSA setting
is not without consequence.
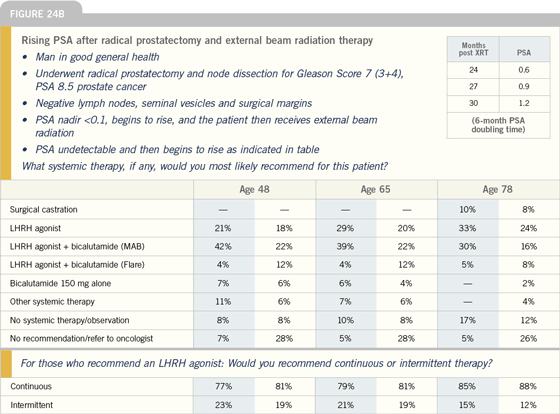
Click here to see image
Defining the optimal time to
initiate hormonal therapy
| Prostate Cancer Update 2005 (1) |
DANIEL P PETRYLAK, MD: Randomized
trial data suggest that earlier hormone therapy is beneficial at the point of PSA
progression, but no data absolutely indicate
benefit in the asymptomatic patient
with a rising PSA. We know from studies
of combination therapy that patients at
high risk will benefit from early hormonal
therapy plus radiation therapy. Ed Messing’s
trial randomly assigned patients
who had positive lymph nodes after
prostatectomy to immediate hormonal
therapy versus delayed hormonal therapy.
The trial demonstrated that earlier
hormonal therapy was beneficial.
A number of important questions
must be answered. Does a threshold
value of PSA need to be defined for
these patients? Does PSA doubling
time depend on regional clinical characteristics?
We need to investigate these
questions.
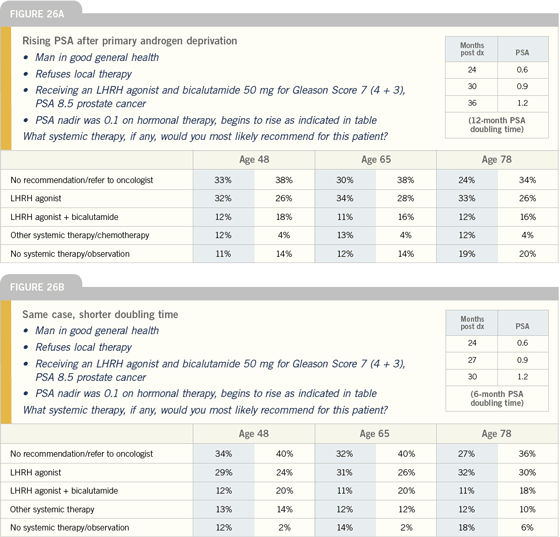
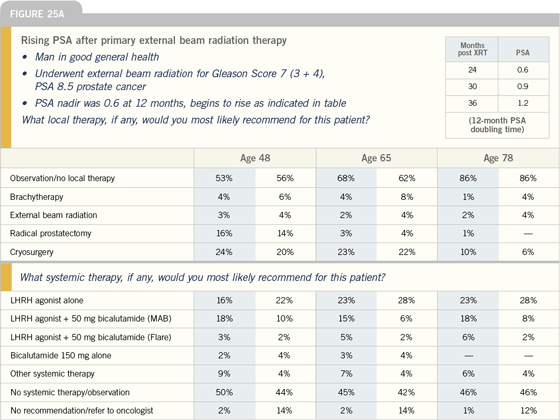
Click here to see image
| Prostate Cancer Update 2005 (2) |
DR DREICER: Earlier versus deferred
hormonal therapy is a major breaking
point in the GU community —
particularly among the zealous believers
in early androgen deprivation and the
more nihilistic among us. In my own
practice, because we see a large number
of patients with biochemical failure, I
have alternative, immunomodulatory
investigational options. Putting that
aside, PSA doubling time is increasingly
useful to predict which patients are more
likely to develop systemic progression in
the hormone-naïve setting.
I discuss the controversies of early
androgen deprivation with patients and
discuss why my colleagues are advocates
of earlier therapy. When the patient asks
me, ultimately, where I stand on the matter,
I tell him that I respect the toxicity
profile of androgen deprivation therapy.
For a long time we have undersold the
impact of androgen deprivation on quality
of life.
I tend to advocate early androgen
deprivation therapy for the motivated
patient with a shortening PSA doubling
time, which sometimes occurs after a
relative period of stability. Now, is that
correct? I don’t know the answer to that
question, but in my practice, that’s the
situation in which I talk to patients in a
more proactive way.
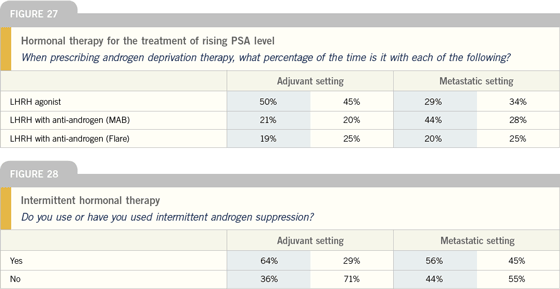
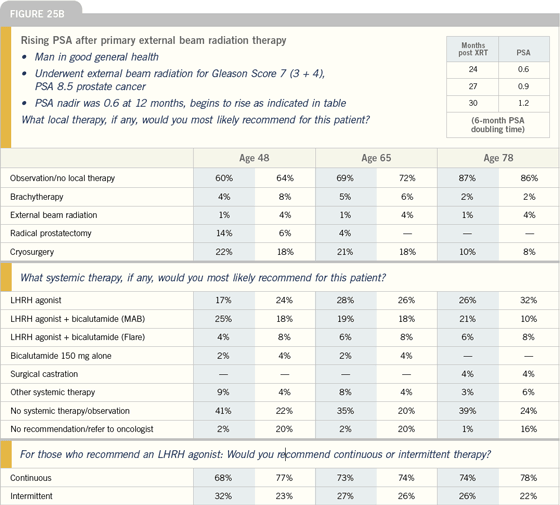
Click here to see image
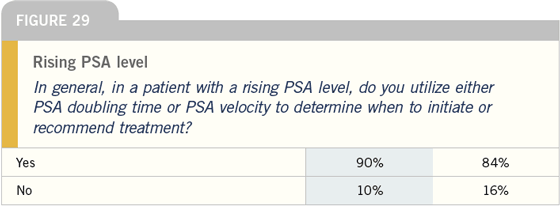
RTOG-9601: Radiation
therapy with or without
bicalutamide 150 mg
| Prostate Cancer Update 2005 (2) |
DR GOMELLA: This Phase III randomized
study is in patients with PSA
relapse following radical prostatectomy.
The study is closed to accrual, and we
are anxiously awaiting the data. This
will be one of the most exciting trials
to be reported because it will determine
whether it’s beneficial to combine
hormonal manipulation with radiation
therapy in the salvage setting.
RTOG-8531 showed that patients
who received radiation and hormones
together after radical prostatectomy for
unfavorable prostate cancer had a survival advantage over patients who only
received radiation therapy. I believe
RTOG-9601 will also be a positive study
because we know the effectiveness of
bicalutamide 150 mg in the adjuvant setting.
Based on the Iverson and See data,
it would be a stretch to think the combination
would not be more effective than
radiation therapy alone.
Bicalutamide 150 mg is approved in
over 50 countries around the world; however,
it has not received FDA approval in
the United States. In Europe, bicalutamide
is commonly used as step-up therapy
in which patients receive oral agents,
such as a 5-alpha reductase inhibitor,
with a small dose of bicalutamide. The
bicalutamide dose is then increased up
to 150 mg before the patients are started
on an LHRH analog as their definitive
therapy.
Currently at our center, the medical
oncologists’ standard salvage regimen for
patients whose disease is failing standard
androgen ablation is bicalutamide 150
mg. We have seen responses to this regimen
last for over a year and a half, so it
appears to be reasonable salvage therapy
and can be offered to patients. It does
appear that a small percentage of men may have an increased cardiac toxicity
associated with the drug. The number of
men who had adverse cardiac outcomes
and the number of increased death rates
in the low-risk arms of the EPC studies
with bicalutamide 150 mg were low,
but noticeable. These findings may have
been statistical aberrations or statistical
noise; nonetheless, they need to be further
examined.
Although bicalutamide 150 mg is not
currently approved for salvage therapy in
the United States, I believe it’s appropriate
to discuss it with patients for whom
it may be suitable, such as those who
are sexually active and want to maintain
their sexual functioning.
Bicalutamide can preserve sexual
function, whereas a high percentage of
men on an LHRH analog therapy experience
significant sexual dysfunction.
Quality of life and determining what’s
important to the patient have become
central issues when considering treatment alternatives in prostate cancer.
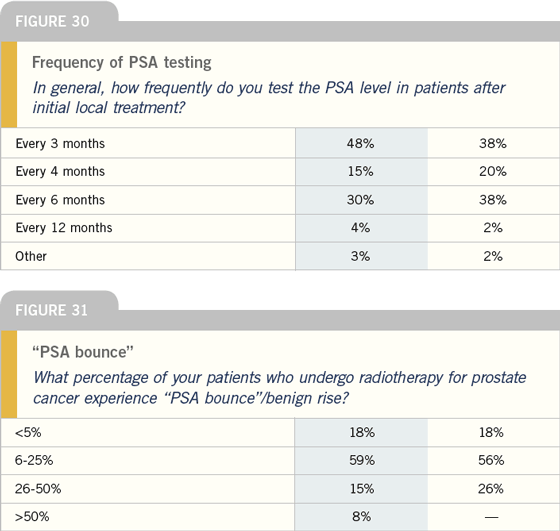
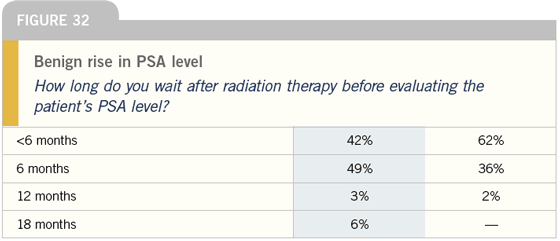
Intermittent androgen
deprivation therapy
| Prostate Cancer Update 2005 (3) |
LAURENCE KLOTZ, MD: I think the role
of intermittent androgen deprivation
therapy for patients with D2 disease
is not that compelling. Of course, the
more common situation is a rising PSA
after the failure of local therapy, and
the majority of these patients are being
treated with hormonal therapy too early
and aggressively. Many of them, such as
a 75-year-old man who received radiation
therapy five years ago and now has a
PSA of 3 ng/mL, are probably not at risk
of death from prostate cancer. Opinions
vary, but my view is that many of these
patients are not at risk. The data are
clear that these patients do not need to
be treated at that point.
If they are going to be treated, however,
the less treatment the better, and
intermittent androgen deprivation therapy
is appropriate, even if the trials show
a modest adverse effect on survival. I
think the patients who are at risk of
not doing well on intermittent androgen
deprivation therapy are those with quite
advanced, relatively rapidly progressing,
life-threatening disease.
Impact of brachytherapy on PSA
| Prostate Cancer Update 2005 (3) |
DR ZIETMAN: We’ve learned that after
brachytherapy, we have to sit on our
hands for three or four years. If the PSA
goes up, we need to ignore it. In fact, we
could make a case for not checking the
PSA at all in the first three years, which
is hard to sell to patients. The median
time to the PSA bounce is about 18
months, and it should be heading down
again within the third or the fourth
year. If it’s not, something is probably
wrong. The PSA after brachytherapy
keeps going down, and at seven or eight
years, the median PSA is lower than at
four or five years.
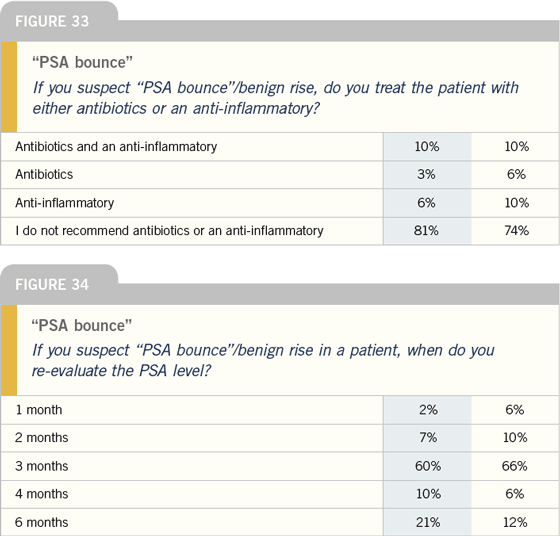
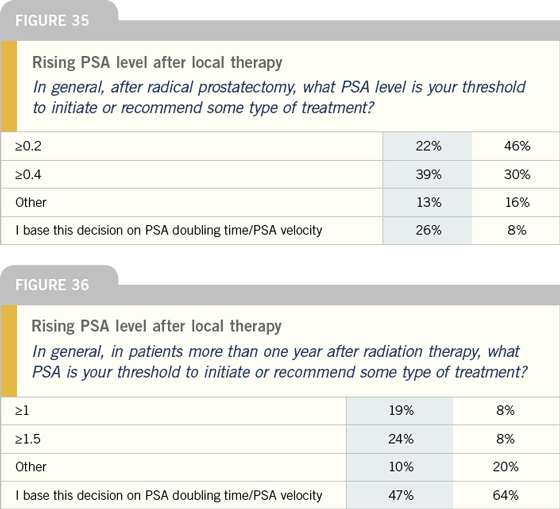
Bicalutamide monotherapy
for rising PSA
| Prostate Cancer Update 2005 (3) |
DR KLOTZ: Certainly, people talk about the
benefit of bicalutamide 150 mg in terms
of sparing libido, but I think the bone
mineral density story is more compelling.
Bicalutamide 150 mg actually increases
bone mineral density because the high
levels of testosterone are converted into
estrogen, which is a bone mineral density-
sparing hormone. To me, that is really
the strong argument for its use.
There are two caveats, however. First
is the question of whether bicalutamide
150 mg is equivalent in terms of duration
of survival. The second issue is gynecomastia.
A number of my patients who
are on the bicalutamide EPC trial have
had breast reduction surgery. They’re
quite happy, but this was definitely an
issue for them.
I have not used much prophylactic
radiation in these patients. I probably
should use more of it, but it doesn’t work
in everyone, and it’s radiation to the chest.
The patients aren’t too keen about it, so
I haven’t really employed it. There are
studies using tamoxifen, but one of the
problems is that if it’s the estrogen that’s
contributing to the increase in bone mineral
density, maybe by using tamoxifen to
block gynecomastia, you’re blocking the
benefit to bone mineral density. From
a theoretical perspective, it is possible
tamoxifen will have an adverse effect.
| Prostate Cancer Update 2005 (4) |
HOWARD I SCHER, MD: Bicalutamide
monotherapy does have some side effects,
in terms of fatigue and gynecomastia,
which can be significantly disfiguring.
In the early 1990s when PSA was starting
to be utilized, we were being referred
patients from our surgical and radiation
oncology colleagues with a rising PSA
alone.
Recognizing that the toxicities of
bicalutamide alone were different, we
actually conducted a study and still have
patients on it from 1993 using bicalutamide
200 mg, not the 150 mg dose, just
trying to see if we could control the disease. We did observe that patients had
disfiguring gynecomastia, in some cases
requiring surgical reduction. But then
again, of the original 50 patients who
had a rising PSA, there were still eight on
therapy in the year 2005. Almost an overwhelming
majority had PSA responses.
Again, this was a 200 mg dose. This is an
option we bring up with patients.
In addition to discussing the gynecomastia
with patients, we also have to
discuss bicalutamide in the context of
the randomized trials, which suggest
that the outcomes may in fact be inferior
to conventional hormones alone in
patients with metastatic disease. What
was of interest in the trial we conducted,
and others as well, was that we’ve looked
at the response in patients who’ve been
on bicalutamide monotherapy and then
cross to a GnRH analog.
The idea being, okay, we’ll protect
your bones. You’ll be stronger while
you’re on bicalutamide, and then we’ll
add the conventional hormone later. It
was only about 30 percent who responded
with the crossover. So it’s clearly a
different drug. Again, we do discuss it,
but I think pound for pound, it’s probably
not equivalent to more conventional
hormones. That said, there are patients
who will opt for it.
Role of chemotherapy in
PSA relapse and locally
advanced disease
| Prostate Cancer Update 2005 (1) |
DR DICKER: I usually refer patients with
PSA relapse and no evidence of skeletal
disease to medical oncologists who
specialize in prostate diseases. I also
encourage them to enroll in clinical
trials that evaluate cytostatic therapy or
some of the anti-androgen-type drugs. I
believe most medical oncologists would
be uncomfortable using cytotoxic therapy
in a patient who does not have a positive
scan. We don’t have any evidence
that simply reducing PSA in a patient
with nonradiographic metastatic disease
has an impact. Chemotherapy has the
potential to harm patients, and we don’t
know the optimal duration for chemotherapy.
We have preclinical data evaluating
the anti-angiogenic effects of
taxanes (both paclitaxel and docetaxel)
in a variety of disease settings. I believe
in the next year or two we’ll see chemotherapy
being combined more frequently
with hormones and radiation therapy
in the locally advanced disease setting.
We all agree that a Gleason eight, nine
or 10 is locally advanced disease, but we
see plenty of tumors with lower Gleason
scores and 15 out of 15 positive biopsies.
I put those patients in a locally advanced
disease category because if they have
surgery they will have positive margins,
and some will have seminal vesicle and
lymph node involvement. It’s a gray area,
but patients with a Gleason seven, PSA
less than 10 and appropriate performance
status may benefit from hormones and
chemotherapy.
| Prostate Cancer Update 2005 (4) |
DR SCHER: Regarding patients with a
rapid PSA doubling time but with PSA-only
disease, I’m not sure we fully know
the natural history of that group. The
tendency has been to use a second- and
third-line hormone therapy first. Again,
that will depend on the initial response,
but I’ve seen situations where the second
hormonal response exceeds the first. It
doesn’t make sense, but that’s what has
happened. There’s a real debate as to
when to play the chemotherapy card.
I could probably count on one hand
the number of times I’ve actually recommended
chemotherapy for this group.
It’s a not a curative treatment, so the real
question is, when do you play that card?
If it were curative, that would be a different
story.
| Prostate Cancer Update 2005 (4) |
DR ROACH: Certain things make me
nervous about not treating somebody
with chemotherapy. If there are pretreatment
high-risk features — a high-grade
tumor, high Gleason score, high PSA,
they were treated and now they’ve failed.
The earlier they failed, the faster their
PSA is rising; these are the issues that
tend to make me want to be more aggressive
with chemotherapy.
In patients who have low-risk features
at the outset — the PSA and stage are
not that high — and they have PSA-only
failure, but their PSA is going up slowly,
I would not recommend chemotherapy,
although it’s possible that they would
benefit. The data for androgen-independent
disease is primarily based on
patients who had extensive experience
with hormonal therapy and had been
through multiple manipulations with
hormone therapy; they also had metastatic
disease, by and large, as opposed to
PSA-only failure. There are studies that
are being contemplated in the RTOG
and other places, based on patients with
PSA-only failure, in which the PSA doubling
time is being incorporated into the
eligibility to try and select out patients
who are at higher risk for death.
Select publications
|

