| |
Chemotherapy for Metastatic Disease |

Click here to see image
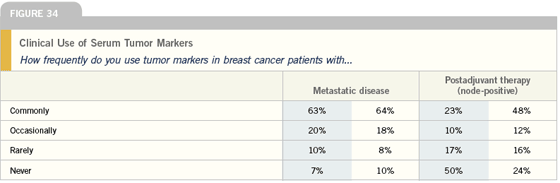
Clinical use of tumor markers
I’ve found tumor markers to be helpful in cases where patients have metastatic disease that’s not readily monitored, easily palpable or visible on chest x-ray. I’ll often order markers on patients who require a bone scan, CT or MRI on a regular basis. This allows me to monitor their response to therapy a little more closely.
Obviously, if the markers are climbing in the face of therapy, then I’ll step back and do the scans and try to see what’s going on in more detail. Using markers allows me, on a treatment-by-treatment basis, to have a reasonably good idea of whether the patient’s responding or progressing in between scans.
However, I try to discourage them in settings where I feel that they will not really help me with clinical monitoring. I have to admit that I have had my arm twisted by patients, as most of us have, to do markers, even after informing them that they are not standard of care or recommended by most professional groups. Patients come in with a high level of anxiety, and I think you have to weigh the patient’s psychology in your decision to do this.
For a patient with metastatic disease who is absolutely, over-the-barrel anxious and can’t be convinced that markers aren’t going to add to her care, I’m willing to do a set of them. If the marker is elevated and I’ve opened Pandora’s box, then I am obligated at some interval to re-check them.
— Gary Lyman, MD, MPH
The ASCO guidelines do not recommend tumor markers in women with node-positive disease after adjuvant therapy. While the use of markers can lead to an earlier diagnosis of metastatic disease, that doesn’t improve survival, which is why the guidelines don’t support their use.
However, patients often request them — especially women with high-risk disease — and I believe there is variability in their use in community practices.
— Joanne L Blum, MD
I am a true proponent of clinical observation, and I don’t use tumor markers, except in patients who have metastatic disease. In node-negative, node-positive or locally advanced disease, I find that tumor markers give patients and physicians false reassurance.
When markers start to rise, there is alarm, often long before you can find evidence of disease recurrence on imaging studies. If the patient very badly wants tumor markers and they’re willing to run the risk, then we do tumor markers. And we’ll follow tumor markers every four to six months.
— Generosa Grana, MD
Click here to see image
We don’t use tumor markers to screen for metastatic cancer after adjuvant treatment. We give patients the ASCO guidelines about the follow-up of patients with early-stage breast cancer and explain that tumor markers really add nothing to their follow-up. Fewer and fewer patients are asking us to look at tumor markers, but when they do, I always tell them, “If you want me to do it, we’ll do it.”
If a patient has a symptom that we think might be metastatic breast cancer, we might order tumor markers, but we generally use them only if a woman has metastatic breast cancer to help us monitor response to therapy.
— Anne Moore, MD
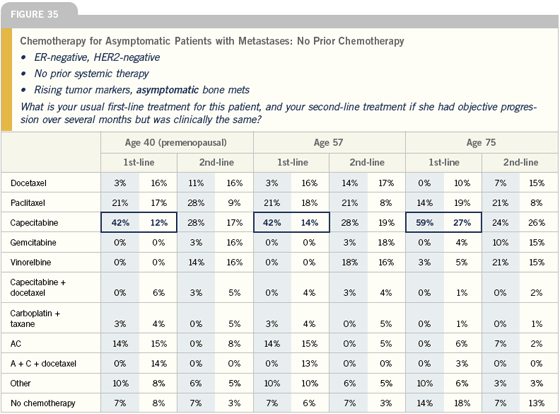
When sequencing single agents, the agent I use depends on which drugs the patient has already received. For example, if a patient with minimal visceral disease has already been exposed to an anthracycline, experienced a long disease-free interval and received hormonal therapy for her metastatic disease, I would consider capecitabine for front-line chemotherapy. I might also consider a weekly taxane first-line.
In an older patient who doesn’t want intravenous chemotherapy and doesn’t want to lose her hair, I’ll certainly consider capecitabine up front, even though it was originally FDA-approved for the anthracycline- and taxane-refractory population.
Capecitabine clearly has activity, is well tolerated and can be used for a long time. In addition, it’s a good transitional agent to use when a patient is being switched from hormonal therapy to chemotherapy — it’s oral and patients don’t have to come to the office for treatments. However, I might also use a weekly taxane first-line.
Second-line, I generally use either gemcitabine or vinorelbine. The sequence varies, and it often comes down to patient preference. Neither of those drugs causes alopecia, nor does doxorubicin HCL liposome injection. In multiple-line therapy, each of these agents has equivalent single-agent activity, and I don’t believe the sequence makes any difference in terms of survival. I believe survival of patients with indolent disease is determined by the biology of their disease, not by which agent we select for second-, third-, fourth- or fifth-line therapy.
— Joanne L Blum, MD
Click here to see image
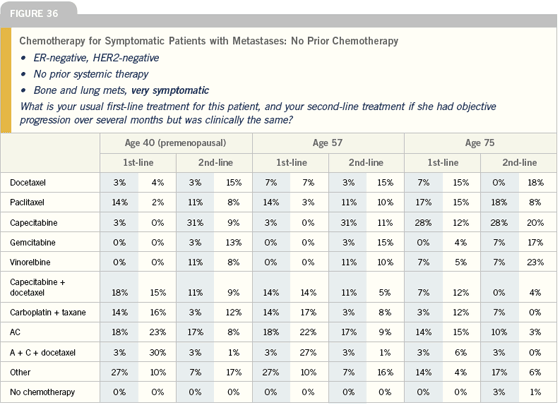
Sequencing of chemotherapy in patients with metastatic disease
There is no set pattern for how to approach chemotherapy for patients with ER-negative, HER2-negative metastatic disease.
In the past, we saw patients who were chemotherapy-naïve, but we almost never do these days. Having “grown up” with and been involved in many different studies, I would probably still use AC in a patient who did not receive adjuvant chemotherapy — assuming she was otherwise healthy with no heart disease.
If she had a response, at some point, I’d stop the doxorubicin and give CMF until the disease progressed.
I would then go on to use paclitaxel, which I feel is probably somewhat better tolerated than docetaxel for very longterm use. If the patient is sicker and needs a more rapid response, I would probably choose docetaxel.
Generally, I would then go to capecitabine. In older women or in those who are very concerned with hair loss, you could certainly make a good argument for starting with capecitabine. It’s a very active drug, as long as the right dose is utilized.
Somewhere along the line, I would also use vinorelbine, gemcitabine and — while I haven’t used it — irinotecan would be on the list now, because it has activity. Liposomal doxorubicin is also an option. We’ve done some studies with this agent, which is always sort of “looking for a home.” You’re going to end up using most of these drugs at one time or another in the treatment of metastatic disease.
In patients who have received adjuvant AC or AC and a taxane, I would start further along in the sequence. If they’ve had AC paclitaxel, then I might use docetaxel, and then go on to the other agents.
— Stephen E Jones, MD
Sequential single agents versus combination chemotherapy
The vast majority of the time, I use single agents in sequence because they are better tolerated. In a younger patient with fairly aggressive disease who wants to be more aggressive with her treatment, with a likelihood of having a somewhat higher response and possibly a survival advantage, and who is less concerned about the toxicity and side effects, I would certainly consider capecitabine/ docetaxel.
I was involved in that study, and this is a very active but fairly toxic regimen. Paclitaxel/carboplatin is pretty well tolerated, particularly on the weekly schedule. But for the most part, I use single agents.
— Stephen E Jones, MD
Click here to see image
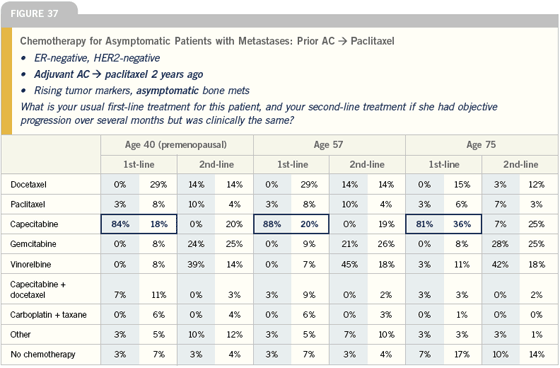
My standard first-line chemotherapy in a nonprotocol situation has been capecitabine. I’ve been impressed by that drug.
Most people have some hormone receptor positivity, and it’s a nice switch to go from hormone pills to chemotherapy pills, as long as you watch the dose and watch for the hand-foot syndrome and diarrhea and have them discontinue therapy if they experience those toxicities before it gets too bad.
I do not use the package insert dose of capecitabine. I use 2,000 mg/m2 per day or 1,000 mg/m2 BID. The question arises: Do you escalate the dose? If they’ve had a nice response and they’re tolerating it well, I might maintain the dose or, occasionally, if they’re in really good shape and have no toxicity at all, I’ll go up a bit higher.
— Charles L Loprinzi, MD
We almost always use sequential singleagent therapy unless the patient is highly symptomatic. We like to start with the oral chemotherapy capecitabine. It’s a very effective drug and patients who have been through adjuvant therapy prefer not to lose their hair or go back on an intravenous regimen.
— Anne Moore, MD
Effects of age on treatment
I try not to let age affect me with regard to treatment recommendations, because the issue is not so much age as it is comorbidities. The data is very solid on this, not only with regard to the benefit of chemotherapy, but also, to a large extent, the toxicity from chemotherapy.
Older women tend to have more complications with any kind of treatment — surgery, radiation or chemotherapy — and may need more aggressive supportive care, particularly with regard to bone marrow function.
However, with appropriate supportive care, these women will respond just as well and the durations of response will be at least as long, if not longer. The toxicities will be manageable and generally not life threatening. So until we are talking about the extremes of age, the issue is more comorbidities.
If a woman has several comorbidities — diabetes, congestive heart failure, a previous stroke and so forth — that woman is at increased risk regardless of her age, and in that case age is just one more comorbidity to add to the bundle. So the bottom line for an otherwise healthy, active older woman who doesn’t have major comorbidities is to treat her exactly as I would a younger patient with the same menopausal status.
With elderly patients, you get into issues of limited life expectancy and, particularly if they’re asymptomatic, that they’re much more likely to die of other problems related to aging. Why add another toxicity, visits to the hospital and a lot of expense, some of which is going to come out of their pocket? For a woman who comes in with symptomatic breast cancer that we would otherwise feel urgently needed to be treated, I will treat. Unless the comorbidities are so imposing and forbidding, I will treat her and use aggressive supportive care to get her through it. She deserves the same opportunity for disease control and longevity as a younger patient.
— Gary Lyman, MD, MPH
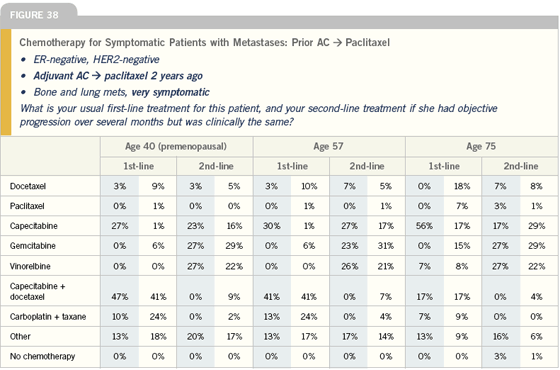
Click here to see image
My choice of chemotherapy is not determined by the patient’s age, but rather the patients’ overall health and any comorbid conditions. A 75-year-old person with no comorbid health problems can tolerate chemotherapy almost as well as a much younger patient. On the other hand, if an elderly patient has significant comorbid health problems, that will impact my selection of therapy.
Renal function is an important consideration, and drugs often have to be adjusted accordingly. The dose of capecitabine has to be adjusted for renal function, particularly in the elderly, and hepatic function is critical when considering a taxane or drugs like vinorelbine. Transportation can also be an issue in selecting therapies. Some patients who live far away or don’t have transportation on a weekly basis may need to receive treatment every three weeks instead. In such cases, transportation may influence the treatment schedule as opposed to which agent is selected.
— Joanne L Blum, MD
Use of nanoparticle paclitaxel
I believe many physicians will now at least consider using nanoparticle paclitaxel where they are currently using paclitaxel. While it does result in neurotoxicity, it is generally very well tolerated with fewer side effects, doesn’t require special tubing or premedication and is a relatively short infusion compared to standard paclitaxel. We have some data on the weekly schedule, which is also very well tolerated.
I believe many of us will probably use nanoparticle paclitaxel in place of paclitaxel in the palliative setting, where it was studied. It has not been compared to docetaxel. So if your choice is docetaxel, because you want to be a little more aggressive to get somebody into remission or whatever the particular reason you’re picking docetaxel, I wouldn’t substitute nanoparticle paclitaxel because we do not have data.
— Stephen E Jones, MD
Most of my experience with nanoparticle paclitaxel has been on protocol; we’ve presented data on two cohorts of patients. At ASCO 2004, we presented data on 106 patients with taxane-refractory disease who received 100 mg/m2 weekly, three weeks on, one week off. We saw a stable disease rate of 15 percent and a response rate of 15 percent, with very acceptable tolerability and safety data. At San Antonio, we presented the second phase of the study with 75 patients who received 125 mg/m2 weekly, three weeks on, one week off. The data was comparable in efficacy and tolerability, although there was a little more sensory neuropathy with the higher dose. Edith Perez will be presenting survival data from her Phase III at the 2005 Miami meeting.
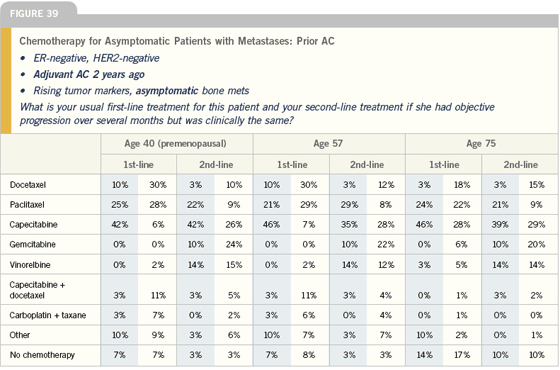
Click here to see image
Nanoparticle paclitaxel received FDA approval in January 2005, and I’ve just started a few patients on it, so I can’t speak to responses in patients off protocol. The data demonstrated that nanoparticle paclitaxel is a superior drug compared to paclitaxel. It’s safer and patients don’t experience hypersensitivity reactions. I believe it will eventually replace paclitaxel. From a taxane-refractory perspective, it clearly has benefit.
Nanoparticle paclitaxel is currently being studied in combination with other agents. We don’t have any safety data yet on it in combination, although we probably can extrapolate from our experience with paclitaxel. In addition, we can examine our taxane-refractory nanoparticle paclitaxel data.
Memorial Sloan-Kettering has an active study evaluating nanoparticle paclitaxel combined with trastuzumab and carboplatin, and Edith Perez has a study examining capecitabine and nanoparticle paclitaxel, but to date no data has been presented or published on combination therapy with this agent.
Other trials are underway as well, evaluating different doses and different schedules with nanoparticle paclitaxel. We’re also moving it to the adjuvant setting, and we’re planning a large clinical trial with dose-dense nanoparticle paclitaxel. It’ll also be studied in other tumors, and we’ll learn the role of this new agent in medical oncology. I believe it’s an improvement in treatment because the drug has proven to be beneficial, patients do not have to take steroids and they don’t run the risk of hypersensitivity reactions.
— Joanne L Blum, MD
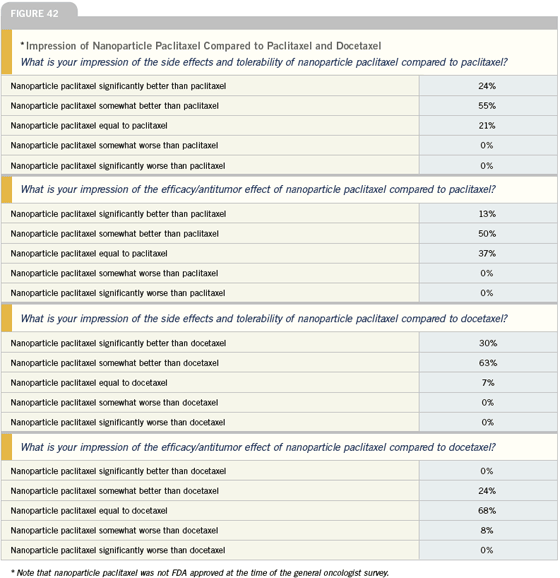
The pivotal trial of nanoparticle paclitaxel in anthracycline-pretreated patients showed it to be as efficacious as docetaxel in terms of response rates. The Phase III trial demonstrated superior efficacy of nanoparticle paclitaxel 260 mg/m2 over paclitaxel 175 mg/m2 in terms of response rate and time to progression.
I believe in the next few years physicians will use nanoparticle paclitaxel for palliation in the metastatic setting in patients whom they want to experience as few side effects as possible. I expect it will be used weekly at 100 mg/m2 for three weeks, followed by one week off, as in Joanne Blum’s study.
I have treated several patients with this agent and found it to be extremely well tolerated, particularly at the 100mg/ m2 dose. I don’t premedicate patients receiving nanoparticle paclitaxel, because patients do not have problems with hypersensitivity reactions. I find weekly dexamethasone is not well tolerated by patients — it tires them and has a crash effect. Avoiding premedication may be one of the reasons we don’t see significant side effects with the nanoparticle paclitaxel.
— Joyce O’Shaughnessy, MD
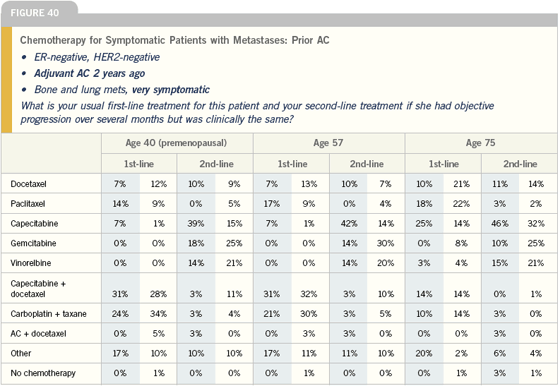
Click here to see image
Nanoparticle paclitaxel is an exciting drug. It’s a product of good pre-clinical work. Now we have a solid Phase III study and two very interesting Phase II studies. I have looked at the data very carefully and discussed it with investigators, and I’m very enthusiastic about helping with future development of this drug and introducing it into our clinical practice.
In the past, in a nonprotocol setting for a patient who is taxane-naïve, we have been using paclitaxel and, on some occasions, docetaxel once every three weeks. But currently, nanoparticle paclitaxel really has to be considered.
The challenge is that we don’t have comparative trials right now of nanoparticle paclitaxel every three weeks versus paclitaxel weekly or of nanoparticle paclitaxel every three weeks versus docetaxel every three weeks. We have different studies showing efficacy, but we lack these head-to-head comparisons of what we think might be the two best approaches with the older taxanes versus nanoparticle paclitaxel.
In this setting, tolerability is paramount to me. Based on the randomized Phase III trial that has been reported, nanoparticle paclitaxel really has a lot of advantages. I’m hesitant to say it is the only taxane I’m going to use, because I think we need more studies. But I don’t think we should wait completely and not use the drug for five years. We really need to start discussing the availability of these agents with all of our patients with metastatic breast cancer.
The nonprotocol situation, patients who have received prior taxane therapy are the perfect patient population to consider for nanoparticle paclitaxel, again, based on the Phase II studies that have been reported to date.
The natural question is: What about the adjuvant setting? It’s very tempting to think about moving nanoparticle paclitaxel into the adjuvant setting, but it’s a pity that we don’t have any data. I hope there will be clinical trials to further explore the optimal way to use this agent. Our cooperative group has not started any trials with nanoparticle paclitaxel in the adjuvant setting, but I’m aware that other groups are developing trials at this time, and I am very supportive of those ideas.
— Edith A Perez, MD
Click here to see image
Click here to see image
We look forward to nanoparticle paclitaxel. The advantages are the short infusion time and lack of allergic response. We have no experience yet, but in metastatic disease, as soon as it’s really available, I believe we’ll be using it.
I also believe the oncology community will embrace it, because we can take a patient who currently spends up to three or four hours of infusion time and administer something that takes a half an hour. That’s very appealing, not only for the patient, but for the doctors who are trying to get patients in and out.
I wouldn’t use it now in the adjuvant setting without data, but I would certainly enter a patient into any available trials.
— Anne Moore, MD
I am thinking of integrating nanoparticle paclitaxel into my practice. I actually treated my first patient with it recently. She had a very poor performance status, had not received chemotherapy and was very symptomatic from her disease. My thinking was to use single-agent paclitaxel or docetaxel, and I chose to use single-agent nanoparticle paclitaxel given on a weekly schedule, because I think the data is very good and the toxicity manageable.
I think that the general oncology community will rapidly accept this agent for a variety of reasons. First, it can be given every three weeks or weekly and the data is good for both. Number two, I think the toxicity profile is favorable. The neuropathy is reversible, the infusion is briefer and there are none of the allergic reactions to the cremophor — all of which are appealing when considering utilizing it.
I am looking forward to those trials that will address the use of nanoparticle paclitaxel in the adjuvant setting, because ultimately we have a drug that appears to be more active than paclitaxel, which has been our mainstay taxane in that setting, and it would be interesting to think that this would give us additional benefit.
— Generosa Grana, MD
Select publications
|

