|

Click here to see image
Algorithm for HER2 testing
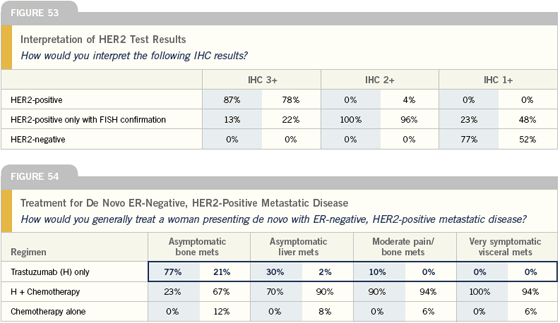
At our institution, we perform IHC testing to assess a tumor’s HER2-status initially. Those that are zero or 1+ are considered negative, and 3+ scores are considered positive. Tumors that are 2+ by IHC are tested further by FISH. This algorithm is endorsed by the College of American Pathologists.
Most of the literature reports that errors in HER2-testing are seen in the tumors that are 2+ by IHC and principally in centers that perform a small number of tests. We have published our pathology results at the Harvard Cancer Center and we have a greater than 90 percent positive and negative predictive value.
With this algorithm, the possibility exists that patients with scores at either end of the spectrum will be under- or overtreated. There probably are a few cases in the zero to 1+ category that are truly HER2-positive by gene amplification. Likewise, probably a couple of percentage of patients whose tumors are 3+ by IHC are really FISH negative, and they may not receive much benefit from trastuzumab, although they might — that has not been studied extensively.
Testing the HER2-status by IHC or FISH is a challenge for many pathology centers, especially small centers that perform a relatively low volume of tests. We have learned from the HER2 story that if we are going to base treatments on the biology of a disease, then we need accurate interpretation of the biology by pathologists. As a result, HER2-testing has improved in the past few years and the pathology community deserves tremendous credit for this. Pathologists recognize the limitations of IHC and, if they see a small volume of cases, they send them elsewhere. In addition, IHC 2+ cases and suspicious cases are retested with FISH.
Click here to see image
I am far more concerned today with the accuracy of estrogen-receptor testing than HER2 testing. We don’t have the same level of quality control in ER-testing, and accuracy is critical when considering endocrine therapy in the adjuvant setting.
— Harold J Burstein MD, PhD
My standard algorithm is to consider tumor specimens that are IHC 0 or 1+ as HER2-negative. There are exceptions to the rules. If the disease is behaving in a particularly malignant fashion, I may want to have FISH testing performed, but that’s not my general practice.
If the tumor is 3+, then I’d be comfortable with calling that positive, and if it’s 2+, then I’ll go ahead and order FISH testing.
— Charles L Loprinzi, MD
In a patient for whom I’m considering trastuzumab, I use FISH testing — I believe that’s the gold standard. For a patient whose tumor is cold negative or in a tumor that is 3+, we probably don’t need FISH. However, results of 1+ and 2+ are indeterminate, and FISH is required. Thirty-five percent of 2+ tumors are FISH-positive. In addition, published data suggests significant observer variability from pathologist to pathologist.
— Joanne L Blum, MD
We use IHC, and generally, if the tumors are 1+ or 3+, we do not request FISH — with some exceptions. In general, they test or recommend FISH for 2+ specimens, if clinically indicated. Most of us do not order FISH for tumors that are 1+ or 3+, unless it doesn’t make sense or feel completely in sync.
For example, if you have a low-grade lobular carcinoma that is 3+, we’ll generally ask for a FISH because you don’t see that very often. Likewise, if it’s a high-grade, nasty looking cancer and it’s 1+, we might consider FISH just to make sure. But most of the cases that we feel compelled to FISH are those read as 2+.
— Ann Partridge, MD
Approach to HER2-positive disease
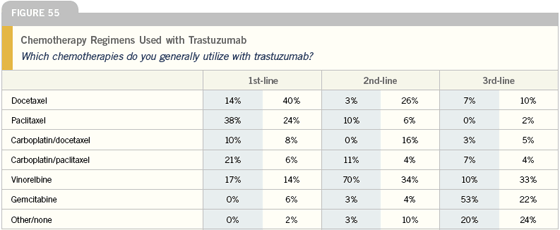
In selecting first-line therapy for patients with HER2-positive metastatic disease, I consider the pace of the disease and the patient’s desires. If a patient can tolerate chemotherapy and has substantial disease in the liver or lungs, I use docetaxel/carboplatin/trastuzumab. In an older woman or a frail patient or a woman who doesn’t want to lose her hair, I select vinorelbine/trastuzumab.
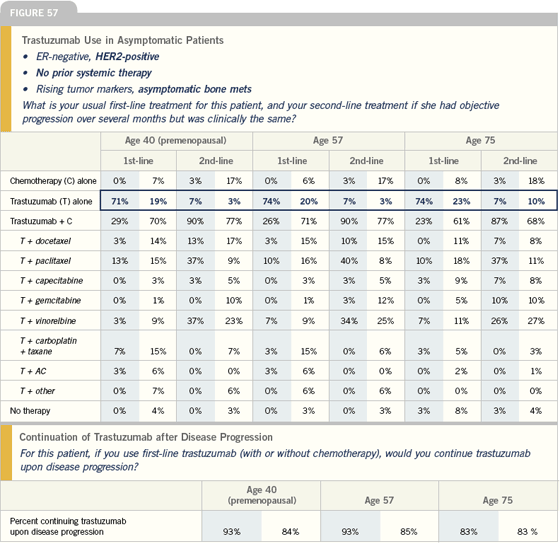
If the patient has ER- and PR-negative disease with only bone or maybe a few soft-tissue metastases, I use trastuzumab alone. In Vogel’s data, approximately 25 to 35 percent of women with metastatic, FISH-positive disease responded to single-agent trastuzumab.
Click here to see image
I’ve also used a combination of capecitabine and trastuzumab in the first-line metastatic setting in select cases. For example, in patients with very high bilirubin levels, I find it difficult to give a taxane or anthracycline. However, an abstract presented at ASCO several years ago showed it was safe to use lower-dose capecitabine in these patients. In vitro data from Slamon and Pegram showed that perhaps these drugs were additive and many clinicians, I believe, overinterpreted that data and felt capecitabine shouldn’t be combined with trastuzumab. I don’t necessarily agree and a number of clinicians, including myself, have had some success with this combination.
— Adam M Brufsky, MD, PhD
I use trastuzumab-based therapy in every patient with HER2-positive metastatic disease by FISH. And I tend to confirm my HER2-positivity by FISH. Only in the patient who is completely asymptomatic with low volume disease do I give trastuzumab alone. The majority of my patients receive trastuzumab with chemotherapy.
— Generosa Grana, MD
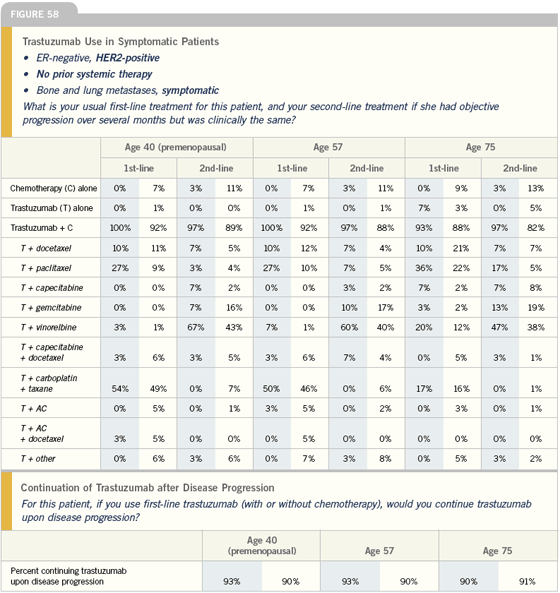
If a symptomatic patient presents with de novo ER-negative, HER2-positive metastatic disease in the bone and lung, I use combination chemotherapy with trastuzumab up front — typically a taxane, carboplatin and trastuzumab.
If she had previously received adjuvant AC, the taxane, carboplatin and trastuzumab combination would, again, be excellent. Capecitabine combined with vinorelbine and trastuzumab would be another interesting combination for such a patient.
For an asymptomatic patient with bone metastases, I have used trastuzumab combined with capecitabine with good results. The other option would be a drug like vinorelbine with trastuzumab or a taxane with trastuzumab, depending on what she previously received.
If a patient is completely asymptomatic, one could use single-agent trastuzumab; however, it’s very rare for a patient with bone metastases to be without symptoms. Generally these patients are experiencing pain.
— Joanne L Blum, MD
Click here to see image
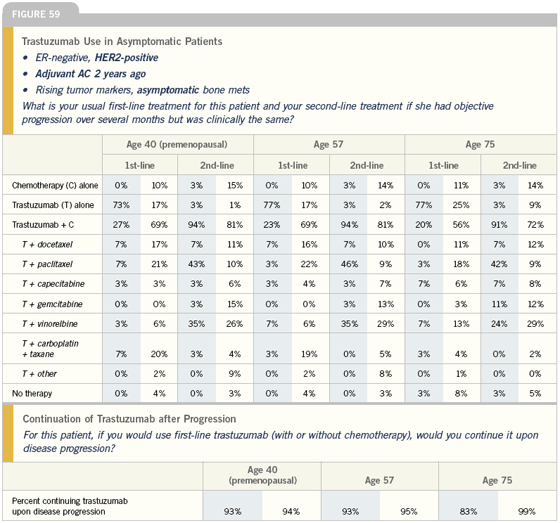
For symptomatic or asymptomatic patients with HER2-positive disease, you might try trastuzumab alone, but the studies revealing a survival benefit with trastuzumab and chemotherapy are pretty compelling, and rarely do you see survival benefits in the metastatic setting. So I usually try to use chemotherapy with trastuzumab in those patients, regardless of symptomatology.
Click here to see image
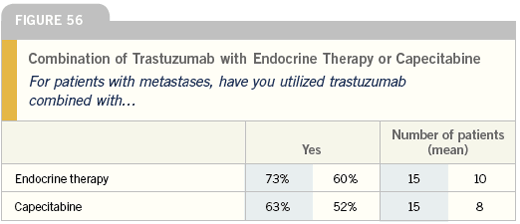
I’ll usually try something gentle, such as vinorelbine/trastuzumab. This regimen is very well tolerated — patients don’t even lose their hair. If someone needs a quick response and they’re chemotherapy- or taxane-naïve, I believe paclitaxel/carboplatin/trastuzumab is a very effective regimen.
We have a neoadjuvant study ongoing that randomly assigns women to vinorelbine/trastuzumab or paclitaxel/ carboplatin/trastuzumab. We don’t know which one’s more effective. They’re both good regimens. Certainly, vinorelbine/ trastuzumab is more tolerable and I would take it any day over TCH. So when all things are equal, I lean towards that just because it’s less of a quality-of-life burden.
— Ann Partridge, MD
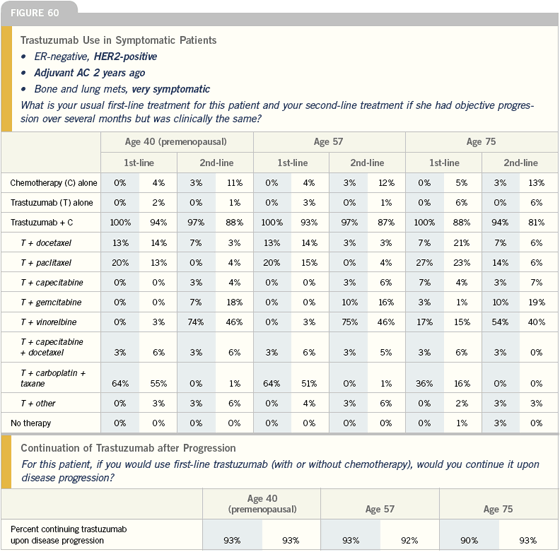
For symptomatic patients with ER-negative, HER2-positive metastatic disease, I offer trastuzumab and chemotherapy, based on the two studies that compared chemotherapy alone versus chemotherapy plus trastuzumab.
Trastuzumab clearly improves survival, and it’s a very important drug for these women. My chemotherapy choice is based on a variety of factors such as side effects. Obviously, I avoid using anthracyclines at the same time as trastuzumab. I commonly use drugs like paclitaxel or vinorelbine — drugs that have been studied and widely reported on in combination with trastuzumab.
— Harold J Burstein MD, PhD
Click here to see image
You can break patients down into two groups: those with relatively indolent versus very symptomatic recurrence. In the former, I’m a big fan of trastuzumab monotherapy up front. Why cause more toxicity than is necessary?
If monotherapy doesn’t go well, I’ll add in chemotherapy — paclitaxel, vinorelbine or the carboplatin/paclitaxel regimen. I had a patient with a fabulous response who is out now to about five years with widespread metastatic disease and doing great, so I’m influenced a bit by that experience.
— Charles L Loprinzi, MD
We certainly incorporate trastuzumab in the first-line treatment of metastatic HER2-positive breast cancer. I generally use a taxane — it’s approved with paclitaxel, but we certainly have good data with docetaxel and frankly, most other drugs that I would want to use in the metastatic setting. There is synergy between trastuzumab and many chemotherapy agents, so with trastuzumab, there’s even more incentive to be giving combination therapy.
If I have a patient with HER2-positive, ER-negative disease that is not life-threatening, depending on their situation, if they’re asymptomatic and have a couple of bone mets, for example, I have used single-agent trastuzumab.
— Julie Gralow, MD
Clinical trials of trastuzumab with vinorelbine
We’ve conducted several Phase II trials, including one at Dana-Farber, in which patients received trastuzumab plus vinorelbine as second- or third-line treatment for metastatic breast cancer. Because of the activity seen, it was moved to first-line where we saw very encouraging response rates on the order of 75 percent with very reasonable time to progression, certainly consistent with other reports of trastuzumab and chemotherapy.
To confirm the data, we then conducted a multi-center Phase II trial, with 17 centers and approximately 50 to 60 patients. Again the response rate was on the order of 70 percent, so we believe this is a very reasonable and active regimen for patients. Since so many patients have received anthracyclines and taxanes in the adjuvant setting, it’s a nice regimen to offer them. Obviously, there are many other regimens that we could use with trastuzumab, this is one we just happen to like.
We conducted a randomized Phase II trial, known as the TRAVIOTA trial, for patients receiving first-line chemotherapy for HER2-positive metastatic breast cancer. All the patients received trastuzumab, and they were randomly assigned to also receive either vinorelbine or a weekly taxane — paclitaxel or docetaxel. Unfortunately, we accrued only approximately 85 patients — far short of our original goal. We are analyzing the data this spring and hope to have the data available around the time of ASCO.
— Harold J Burstein MD, PhD
Trastuzumab scheduling
I generally schedule trastuzumab to accommodate the patient’s chemotherapy schedule. If the patient is receiving weekly chemotherapy, I use weekly trastuzumab. If the chemotherapy schedule is every three weeks, I am comfortable using trastuzumab that way as well. For many of my patients with a great response to trastuzumab plus chemotherapy, I administer the chemotherapy for many months and when they seem to have reached an optimal response point or plateau, I discontinue the chemotherapy and continue with trastuzumab alone. At that point, I often switch patients on weekly trastuzumab to an every three-week schedule, because it’s more convenient for the patient.
— Harold J Burstein MD, PhD
Click here to see image
I don’t see any difference between the weekly and three-weekly schedules of trastuzumab. If patients are receiving weekly vinorelbine, then they’ll receive trastuzumab. If they’re receiving every three-week chemotherapy or trastuzumab monotherapy, I’ll utilize the three-weekly schedule.
— Charles L Loprinzi, MD
Continuation of trastuzumab after progression
The duration of trastuzumab in metastatic disease has not been studied in a randomized trial, so we are conducting an observational study of 400 patients in approximately 50 centers, and every three months we’re recording each patient’s treatment. I expect we’ll find that about 35 percent of clinicians don’t continue trastuzumab after progression. Many believe that progression with a chemotherapy- trastuzumab regimen indicates resistance to trastuzumab, but I don’t agree.
I believe it is beneficial to continue trastuzumab beyond an initial progression, but I don’t know for how many progressions it continues to be advantageous. In our retrospective analysis of approximately 200 patients who received front-line trastuzumab, those who continued on trastuzumab seemed to have a small benefit, at least in time to progression, compared to those who did not. A retrospective study from the Hellenic Cooperative Oncology Group demonstrated time to progression intervals of three to four months with third- and fourth-line trastuzumab plus chemotherapy.
— Adam M Brufsky, MD, PhD
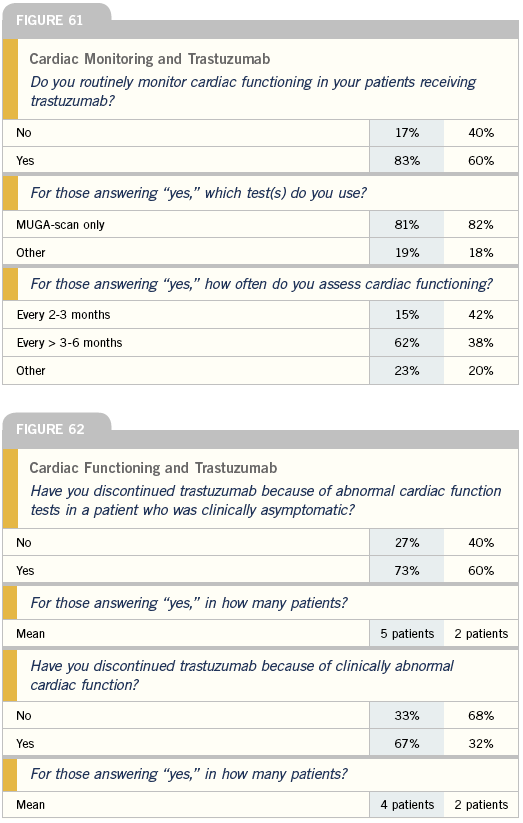
Monitoring for cardiotoxicity with trastuzumab After the initial experience with significant cardiotoxicity in patients on a combination of trastuzumab and anthracyclines, everybody stopped using trastuzumab in combination with anthracyclines, and most of the cardiotoxicity problem went away. For other chemotherapy regimens, whether it’s taxanes or vinorelbine or others, the cardiac toxicity rate is not zero, but it’s considerably less than five percent.
Generally, I order a MUGA or LVEF when I start the trastuzumab and then repeat it approximately roughly four months later. We studied this strategy prospectively in one of our vinorelbine/ trastuzumab trials and showed that none of the patients who had well-preserved LVEF at 16 weeks on therapy developed a significant decline in LVEF. I also check it again when I switch from one trastuzumab-based regimen to another and whenever I’m discontinuing trastuzumab and reintroducing an anthracycline.
The good news is that we believe by avoiding the combination of anthracyclines and trastuzumab, we’ve managed to avoid most of the problem. However, we have to remain respectful of the drug.
The NSABP adjuvant trial data showed a cardiac toxicity rate of approximately four percent in patients who received AC followed by paclitaxel and trastuzumab, versus approximately one percent in patients who did not receive trastuzumab.
I believe this will probably not prohibit the development of trastuzumab in the adjuvant setting, but it is a reminder that it’s not totally benign and should not be used as adjuvant therapy off protocol until we have the clinical data that it is useful in that setting.
— Harold J Burstein MD, PhD
I monitor MUGA scans in my patients on trastuzumab every three months continuously for cardiac toxicity. I believe we need data long-term to tell us whether there’s a point at which to stop monitoring, where you don’t see any continued risk.
— Generosa Grana, MD
I always do a baseline MUGA scan to assess baseline left ventricular ejection fraction. But from there on, in the metastatic setting, if the patient doesn’t have a lot of risk factors for heart disease, I don’t regularly monitor asymptomatic patients without major cardiac risk factors.
I don’t routinely evaluate ejection fractions in asymptomatic patients, because if the patient has a drop in ejection fraction but is asymptomatic, it isn’t going to influence my treatment. I’m not going to stop a drug that the patient’s doing well on. I’m not recommending that my colleagues and my referring physicians do that, but that’s just my practice management style.
I have had patients whose ejection fraction has dropped, but at the first sign of symptoms, I’ll repeat the MUGA and hold the drug until the heart failure is treated.
— Julie Gralow, MD
Endocrine therapy with trastuzumab
In my patients with ER-postive, HER2-positive metastatic disease, I tend to combine an aromatase inhibitor and trastuzumab. However, I am perfectly honest with patients that I don’t know whether this approach gives us additional benefit above and beyond what they could achieve with an aromatase inhibitor alone.
— Generosa Grana, MD
It’s very frustrating that we don’t have a lot of data on the hormonal agents with trastuzumab, but there’s clearly nothing preclinically or, to date, clinically, that would suggest any negative interaction. If anything, given the ER and HER2 signaling pathways, you would hypothesize potential synergy between a hormonal agent and trastuzumab. In my patients with hormone receptor-positive patients, I want to avoid chemotherapy for as long as possible, and I’ll generally start with a hormonal agent.
Frankly, although this is where there’s a difference between the art of medicine and the science of medicine, I will start trastuzumab at the same time as the hormonal therapy, looking for the best blockage of signaling in my breast cancer patients. That’s different than some of my colleagues, and it’s not pure. It doesn’t have the good clinical trials behind it, but I do think that it’s kind of hitting two pathways at once and it makes sense to me to try to combine both of these targeted therapies.
— Julie Gralow, MD
Use of adjuvant trastuzumab
I hope that the adjuvant trials will be positive in the future, but in the adjuvant setting right now, I have a strong bias against the nonprotocol use of trastuzumab. If my patients went on the adjuvant trial, I use the rationale that they would be randomly assigned to potentially not receive trastuzumab — a one-third chance of that for the current NSABP trial.
I also take comfort that the NSABP trial has 3,000 patients enrolled, and they’re out to two or three years. There’s a data monitoring committee watching that trial very closely, and the tendency these days is, if the curves have split and there’s clearly an advantage, a public announcement is made. That gives me some comfort.
Ten years ago, I was pretty sure that high-dose chemotherapy with bone marrow transplant would have beaten standard chemotherapy, and in fact, it didn’t. So given all that information, my bias is not to use adjuvant trastuzumab.
Inflammatory breast cancer is an active disease, with the cancer clearly present, and there’s no clinical protocol evaluating the role of trastuzumab in these patients. I believe most of us would consider it reasonable to utilize trastuzumab in those patients with HER2-positive inflammatory tumors.
— Charles L Loprinzi, MD
Off protocol, I use adjuvant trastuzumab in patients with locally advanced and inflammatory disease. These are women whose disease is inoperable at presentation, who have large, bulky tumors or inflammatory disease.
In these cases, I utilize four to six months of trastuzumab-based chemotherapy, send them to surgery if the disease becomes surgically resectable, and then continue chemotherapy plus trastuzumab. Ultimately, these women receive a year plus of trastuzumab. I am not using trastuzumab-based therapy based on node-positivity alone.
— Generosa Grana, MD
I think — especially based on presentations of some neoadjuvant data now — we’re probably going to see that trastuzumab is a useful drug in the adjuvant setting. The first interim analyses are going to occur for most of the four international adjuvant trastuzumab trials within the next year. And if there is a marked difference between the arms, we may see reporting of at least one of these trials within the next year. If the difference is small, we’ll have to wait for more events to occur.
— Julie Gralow, MD
Select publications
|

